-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Cook Medical COKG19891BX - BALLOON, CERVICAL RIPENING, J-CRBS-184000, EACH
Cook Cervical Ripening Balloon with Stylet
The Cook Cervical Ripening Balloon is a silicone double-balloon catheter with an adjustable-length malleable stylet. It is intended for mechanical dilation of the cervical canal prior to labor induction at term when the cervix is unfavorable for induction.
| Order Number | Reference Part Number | Fr | Length (cm) | Balloon Volume mL |
| G19891 | J-CRBS-184000 | 18 | 40 | 80 |
Intended Use
The Cook Cervical Ripening Balloon is indicated for mechanical dilation of the cervical canal prior to labor induction at term when the cervix is unfavorable for induction.
Contraindications
- Patient receiving or planning to undergo exogenous prostaglandin administration
- Placenta previa, vasa previa, or placenta percreta
- Transverse fetal orientation
- Prolapsed umbilical cord
- Prior hysterotomy, classic uterine incision, myomectomy or any other full-thickness uterine incision
- Pelvic structural abnormality
- Active genital herpes infection
- Invasive cervical cancer
- Abnormal fetal heart-rate patterns
- Breech presentation
- Maternal heart disease
- Multiple gestational pregnancy
- Polyhydramnios
- Presenting part above the pelvic inlet
- Severe maternal hypertension
- Any contraindication to labor induction
- Ruptured membranes
Precautions
If fetal membranes rupture spontaneously while this device is in place, it is recommended that both balloons be deflated and the device removed in preparation for spontaneous active labor contractions.
Potential Adverse Events
Risks associated with use of the Cook Cervical Ripening Balloon and labor induction may include, but are not limited to:
- Placental abruption
- Uterine rupture
- Spontaneous rupture of membranes
- Spontaneous onset of labor
- Device expulsion
- Device entrapment and/or fragmentation
- Maternal discomfort during and after insertion
- Failed dilation or need for caesarean delivery
- Cervical laceration
- Bleeding
- Risk of pre-term labor and birth in subsequent pregnancy
The Cervical Ripening Balloon
- Does not require traction.
- Creates steady pressure on the internal and external os throughout the dilation process.
- Has been shown to improve Bishop scores in nulliparous women in comparison to 30 mL Foley balloon catheters.1
- Is a completely mechanical dilation method.
- Ceases its mechanical action when the device is removed.
- Is associated with reduced rates of tachysystole and increased rates of vaginal delivery within 24 hours in comparison to prostaglandin.
- Has a stylet that is completely contained within the catheter.
Instructions for Use
- Loosen the fitting on the proximal hub of the stylet and adjust the wire so that the distal tip of the stylet is even with the distal tip of the Cervical Ripening Balloon.
- Tighten the fitting so that the wire does not move during manipulation, and seat the adjustable handle firmly into the blue port labeled S.

- Use the stylet with the Cervical Ripening Balloon to traverse the cervix.
Note: Once the cervix has been traversed and the uterine balloon is above the level of the internal uterine opening (internal os), remove the stylet before further advancing the catheter.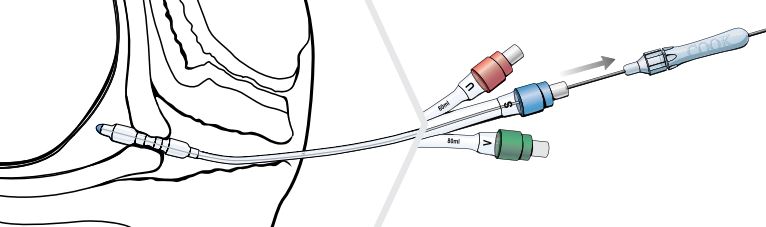
- Advance the Cervical Ripening Balloon through the cervix until both balloons have entered the cervical canal.
- Inflate the uterine balloon with 40 mL of saline. Once the uterine balloon is inflated, pull the device back until the balloon abuts the internal cervical os.
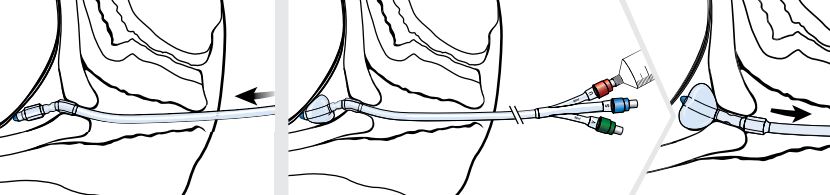
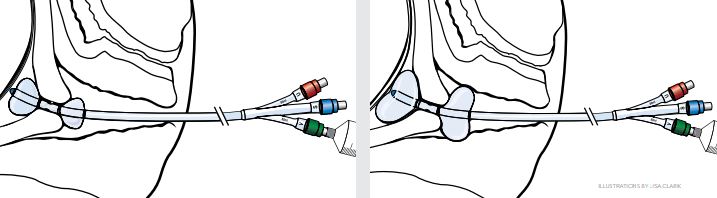
- The vaginal balloon is now visible outside the external cervical os and should be inflated with 20 mL of saline.
- Once the balloons are situated on each side of the cervix and the device has been fixed in place, add more fluid to each balloon in turn, until each balloon contains a maximum of 80 mL of fluid. Time the balloon placement so that the balloon is in place no longer than 12 hours before active labor is induced.
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
How Supplied
Supplied sterilized by ethylene oxide gas in peel-open packages. Intended for one-time use. Sterile if package is unopened or undamaged. Do not use the product if there is doubt as to whether the product is sterile. Store in a dark, dry, cool place. Avoid extended exposure to light. Upon removal from the package, inspect the product to ensure no damage has occurred.



