-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Orasure Technologies 1001-0180 - OraQuick HCV Rapid Antibody Test 100/BX
OraQuick HCV Rapid Antibody Test
The OraQuick HCV Rapid Antibody Test is a manually performed, visually read immunoassay for the qualitative detection of HCV antibodies in human fingerstick and venipuncture whole blood. The OraQuick HCV Rapid Antibody Test is comprised of both a single-use test device and vial containing a pre-measured amount of a buffered developer solution. The test consists of a sealed pouch with two separate compartments for each component. The OraQuick HCV Rapid Antibody Test utilizes a proprietary lateral flow immunoassay procedure.
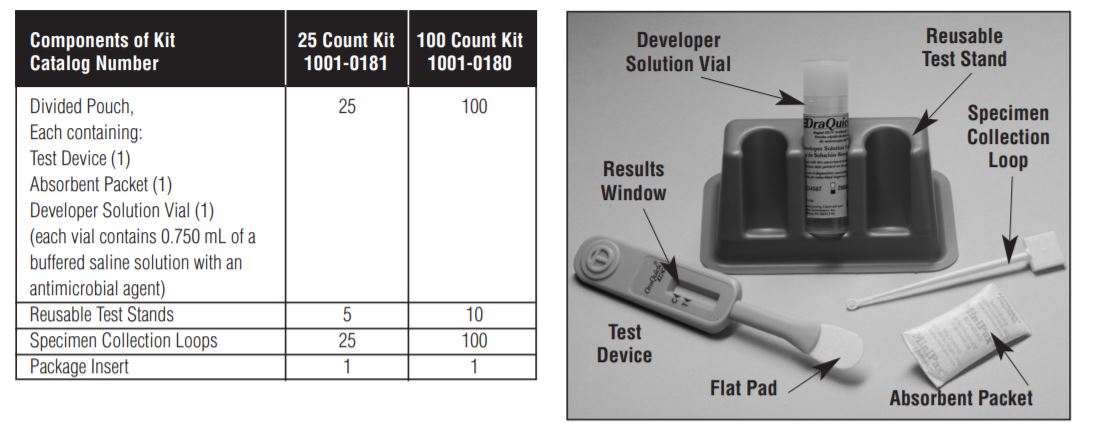
The assay test strip, which can be viewed through the test device result window, contains synthetic peptides and recombinant proteins from the core, NS3, and NS4 regions of the HCV genome (test) and a goat anti-human IgG (procedural control) immobilized onto a nitrocellulose membrane at the Test (T) and the Control (C) Zone, respectively.
A fingerstick whole blood specimen or venipuncture whole blood specimen is collected using a specimen loop and transferred into the developer solution vial, followed by the insertion of the device. The developer solution facilitates the capillary flow of the specimen into the device and onto the assay strip. As the specimen flows through the device, antibodies from the specimen are bound to the protein A gold colorimetric reagent present on the assay strip. If the specimen contains anti-HCV antibodies, the resulting labeled complexes contain HCV antibody and bind to immobilized HCV antigens at the HCV Test Zone (T Zone) resulting in a reddish-purple line. If the specimen does not contain anti-HCV antibodies, the labeled complexes do not bind at the HCV Test Zone and no line is observed in the T Zone. The intensity of the line color is not directly proportional to the amount of HCV antibody present in the specimen. The remaining labeled complexesaretransported to the Control Zone(C Zone) binding to a goatanti-human antibodyfragment. The presence of IgG antibodies in the sample (regardless of their specificity) results in a reddish-purple line at the C Zone. This procedural control serves to demonstrate that a specimen was added to the vial and that the fluid has migrated adequately through the device. A reddish-purpleline willappearat the C Zone during the performance ofallvalid tests; whether or not thesampleis positive or negative for HCV antibodies (refer to the Test Result and Interpretation of Test Result section in this package insert).
Thetest resultsareinterpreted after 20 minutes, but not morethan 40 minutesfollowing theintroduction of the deviceinto the developer solution vial. No precision pipetting, pre-dilutions, or specialized instrumentation are required to perform the OraQuick HCV Rapid Antibody Test.

Orasure Technologies #1001-0181, OraQuick HCV Rapid Antibody Test 25/BX
$585.05 per BOX

Orasure Technologies #1001-0181, OraQuick HCV Rapid Antibody Test 25/BX
$638.05 per BOX

Orasure Technologies #1001-0182, OraQuick HCV Ab Control Positive/ Negative Rapid f/ Analyzer 2/Bx
$74.66 per BOX

Orasure Technologies #1001-0181, ANTIBODY TEST, ORAQUICK HCV RAPID (25/KT)
$596.59 per KIT

