-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Carefusion 260100 - SWABSTICK, CHLORAPREP, 1.75 ML, SINGLE, 480/CS
ChloraPrep Swabstick 1.75 mL Applicator (single)
The ChloraPrep swabstick promotes aseptic surgical skin prep technique prior to surgery. The easy-to-use foam tip is saturated with the clear ChloraPrep solution.ChloraPrep Swabstick -1.75 mL Applicator (Single): 260100 5.25 mL Applicator (Triple). The easy-to-use foam tip is saturated with the clear ChloraPrep solution.
- Patient preoperative skin preparation.
- 2% chlorhexidine gluconate (CHG) and 70% isopropyl alcohol (IPA).
 |  |
Product Benefits
- Rapid acting: ChloraPrep exhibits better immediate antimicrobial activity than povidone iodine alone.
- Persistent: ChloraPrep skin antiseptic maintains antimicrobial activity for at least 48 hours.2 Free iodine, the active ingredient in povidone iodine and other iodophors, has minimal residual activity.
- Broad spectrum: ChloraPrep antimicrobial activity is effective against a broad spectrum of microorganisms on the skin
- Active in protein-rich biomaterials: Remains active in the presence of blood, serum and other protein-rich biomaterials
- Aseptic technique: The patented ChloraPrep applicator promotes aseptic technique and reduces th e risk of direct hand-to-patient contact, helping reduce the risk of cross-contamination
- Friction scrub: ChloraPrep applicators promote gentle friction scrub to help the solution penetrate the first five layers of the stratum corneum, where 80% of skin-dwelling microorganisms reside.
- Latex-free
Indication to use
- For the preparation of the patient's skin prior to surgery or injection. (Single).
- Do not use with electrocautery.
- Do not allow the solution to pool.
- Remove wet materials from the prep area.
- Use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
- Discard the applicator after a single use.
- Note that the applicator is latex-free and for external use.
- Use in a well-ventilated area.
- Do not use for lumbar puncture or in contact with the meninges.
- Do not use on open wounds or as a general skin cleanser.
- Do not use on patients with known allergies to CHG or IPA.
- Keep the solution out of the eyes, ears and mouth.
- Store between 15 to 30 C (59 to 86 F).
- Avoid freezing and excessive heat above 40 C (104 F). Store within the recommended conditions to maintain the efficacy.
Application instructions
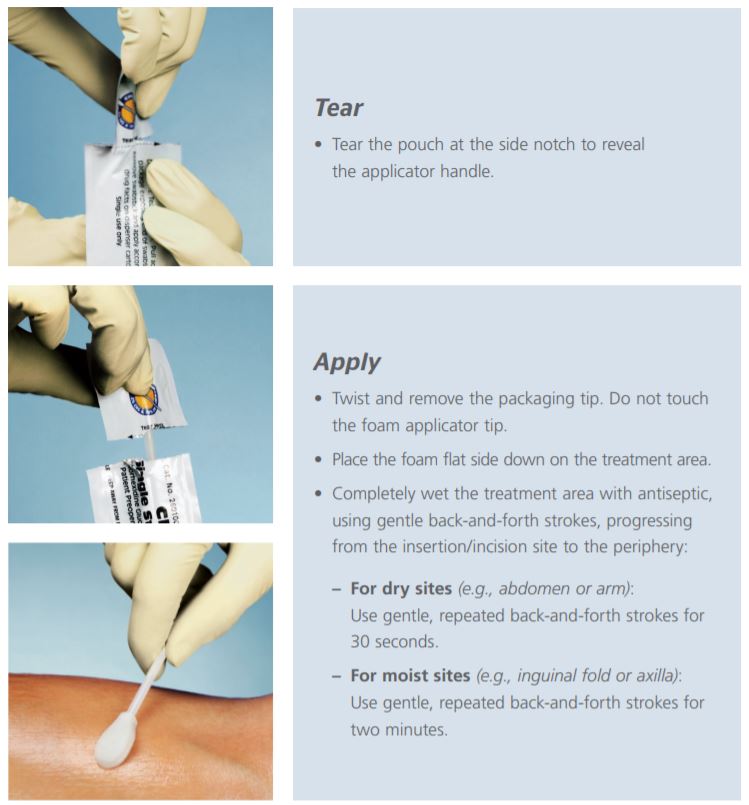
Before using the ChloraPrep swabstick applicator, read the instructions on the package. Use in accordance with the policies and procedures of your hospital.

FAQs
1. What is ChloraPrep preoperative skin preparation?
A rapid-acting and persistent preoperative skin preparation. Its proven formulation is available in six unique applicators designed for procedures ranging from peripheral IV insertion to major surgery.
2. What are the active ingredients of ChloraPrep preoperative skin preparation?
Chlorhexidine gluconate (CHG) 2% w/v and isopropyl alcohol (IPA) 70% v/v.
3. Is ChloraPrep preoperative skin preparation FDA approved?
Yes. After submitting a new drug application (NDA) to the Food and Drug Administration (FDA), ChloraPrep preoperative skin preparation received FDA approval in 2000.
4. Which CDC guidelines recommend the use of a 2% chlorhexidine solution for skin antisepsis?
ChloraPrep preoperative skin preparation meets the Centers for Disease Control and Prevention (CDC) Guidelines for the Prevention of Intravascular Catheter-Related Infections, published in 2002. The guidelines state to "clean skin with a > 0.5% chlorhexidine preparation with alcohol before central venous catheter and peripheral arterial catheter."
5. What category of CDC recommendations does 2% CHG receive?
ChloraPrep preoperative skin preparation meets the Centers for Disease Control and Prevention (CDC) Guidelines for the Prevention of Intravascular Catheter-Related Infections, published in 2002. The guidelines state to "clean skin with a > 0.5% chlorhexidine preparation with alcohol before central venous catheter and peripheral arterial catheter."

BD #260100, ChloraPrep Swabstick Applicator 1.75 ML, Single, 48/BX, 480/CS
$560.40 per CASE

BD #260100, SWABSTICK, CHLORAPREP, 1.75 ML, SINGLE, 480/CS
$549.50 per CASE

BD #260100, SWABSTICK, CHLORAPREP, 1.75 ML, SINGLE, 480/CS
$599.50 per CASE

BD #260100, SWABSTICK, CHLORAPREP, 1.75 ML, SINGLE, 480/CS
$62.82 per BOX

