-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

TrueCare Biomedix TCBINF022AE - 92" GRAVITY ADMIN SET W/ 0.2 MICRON GVS SPEEDFLOW INLINE FILTER, 1 Y-SITE, UNIV SPIKE, 20-DROP FILTERED (15 MICRON) DRIP, ROLL CLAMP, MALE L/L GRAVITY ADMIN SET, 50/CS
IV Administration Set with GVS Speedfow 0.2 Micron Filter
Our IV Administration Set with a 0.2 Micron Filter consists of a universal spike, male swivel luer lock, roller clamp, drip chamber with a 15 micron filter, and a .02 micron in line filter. Each set is individually blister-packaged in quantities of 50 per box and features patient information directly on the exterior of the package.
Features
- 20 drops/ml
- One (1) Y-injection site 6 from distal end
- Male swivel luer lock connector
- Universal vented/non-vented spike
- Roller clamp
- 15 micron filter located in drip chamber for added protection
- 0.2 micron GVS Speedflow in line Filter
- 92" length
- DEHP-free
- Latex-free
Device Characteristics
- What MRI safety information does the labeling contain? Labeling does not contain MRI Safety Information
- Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): No
- Device labeled as "Not made with natural rubber latex": No
- For Single-Use: Yes
- Prescription Use (Rx): No
- Over the Counter (OTC): No
- Kit: No
- Combination Product: No
- Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): No
Sterilization
- Device Packaged as Sterile: Yes.
- Requires Sterilization Prior to Use: No.
Roller clamp
Roller clamp equipped with a small roller that may be rolled counterclockwise to close off primary IV tubing or clockwise to open it. The roller clamp may also be manipulated to increase and decrease the flow of the IVsolution and is easily moved with the thumb, thus making it a one-handed convenience in the administration of IV therapy.
Why Universal Spike?
A vented/non-vented or "universal" spike allows an administration set to be used with all types of solution containers. The vent should be open when infusing from non-collapsible containers (glass and semi-rigid plastic) and closed when infusing from collapsible plastic containers (bags).
GVS Speedflow 0.2 Micron Filter
Membrane filtration
Filtration through a membrane means that the filter material will stop particles larger than the pore size rating. This enables an absolute pore size rating for the membranes for which they are clearly classified. Bacterial retention claims can be made based on the pore size of the membrane.
Pore size and challenge organism
- 0.2 micron => Brevundimonas diminuta
Flow Rate 0.2 Micron Filter
Flow rate comparison for TPN solution through 0.2 micron IV filters.
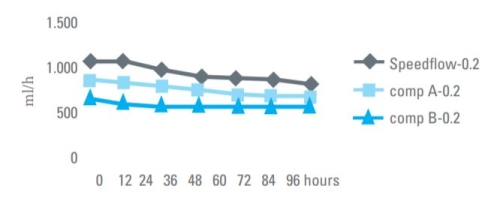
High flow rate, longer life time!
Thanks to the special design of our housing and the structure of our HI-FLO PES membrane, Speedflow IV filters have high flow rates, which relates to longer product life before plugging. Under most circumstances, Speedflow will maintain flow without degradation over 120 hours of administration.
DEHP Free
Our IV sets are DEHP free. Infusion Therapy Standards of Practice advise to usee administration sets free of di-ethylhexyl-phthalate (DEHP) to administer lipid-based infusates, such as IVFE or TNA. DEHP is lipophilic and is extracted into the lipid solution with commonly used polyvinyl chloride administration sets and containers. DEHP is considered a toxin, and studies have demonstrated increased DEHP levels in lipid solutions, which is especially a risk with neonatal, pediatric, and long-term home care patients (42).

TrueCare Biomedix #TCBINF033G, 92" GVS EASYDROP GRAVITY FLOW REGULATOR SET, 1 Y-SITE, UNIV SPIKE, 20-DROP FILTERED (15 MICRON) DRIP, ROLL CLAMP, MALE L/L GRAVITY ADMIN SET, 40/BX
$134.42 per CASE

TrueCare Biomedix #TCBINF001, 92" GRAVITY ADMIN SET, 1 Y-SITE, UNIV SPIKE, 20-DROP FILTERED (15 MICRON) DRIP, ROLL CLAMP, MALE L/L GRAVITY ADMIN SET, 50/CS
Call for Pricing

TrueCare Biomedix #TCBINF044G, 92" GRAVITY ADMIN SET W/ GVS EASYDROP FLOW REGULATOR, GVS SPEEDFLOW 0.2 MICRON FILTER, 1 Y-SITE, UNIV SPIKE, 20-DROP FILTERED (15 MICRON) DRIP, ROLL CLAMP, MALE L/L GRAVITY ADMIN SET, 40/BX
$200.66 per BOX

TrueCare Biomedix #TCBINF033G, 92" GVS EASYDROP GRAVITY FLOW REGULATOR SET, 1 Y-SITE, UNIV SPIKE, 20-DROP FILTERED (15 MICRON) DRIP, ROLL CLAMP, MALE L/L GRAVITY ADMIN SET, 40/CS
$134.42 per CASE

