-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Cook Medical G44783 - Entuit Gastrostomy BR Balloon Retention Feeding Tube with ENFit Connection - 18 Fr Catheter, 10 cm Marked Length, 5-10 mL Balloon Volume, Medium Set Size Used with Entuit Start Initial Placement Gastrostomy
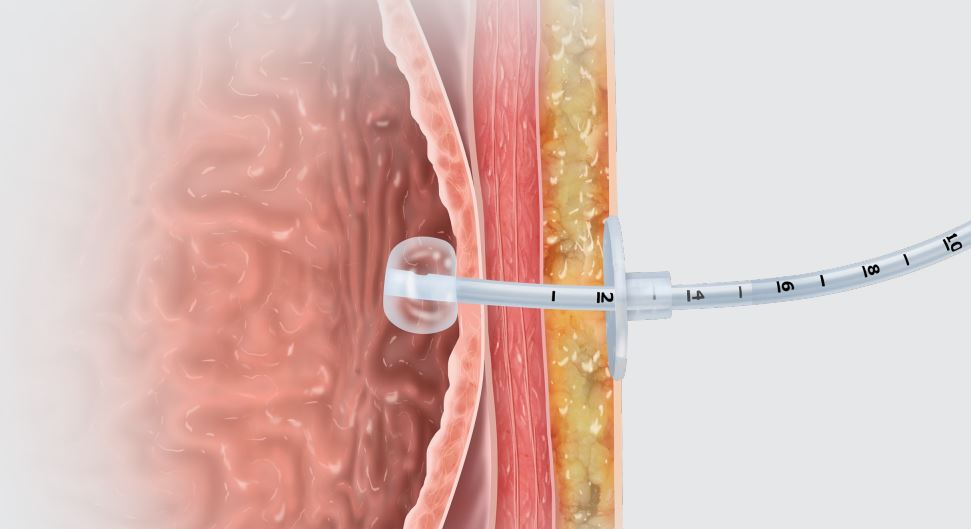
Entuit Gastrostomy BR Balloon Retention Feeding Tube
The Entuit Thrive Balloon Retention Gastrostomy Feeding Tube is a sterile device consisting of a silicone balloon, bi-lumen shaft, three-port funnel and bolster that allows for proper retention during enteral feeding, medication administration and decompression. The device contains one valve that allows for inflation and deflation of the silicone balloon.
Entuit Gastrostomy BR Balloon Retention Feeding Tube with ENFit Connection
| Order Number | Reference Part Number | MR Status | Catheter Fr | Marked Length cm | Balloon Volume mL | Used with Entuit Start Initial Placement Gastrostomy Set sizes |
| G44783 | SBRD-18-ENF | MR Conditional | 18 | 10 | 5-10 | Medium |
Used to provide gastric access for enteral feeding, medication administration, and decompression through an established gastrointestinal stoma tract.
- The unique, integrated design of the in-line balloon shaft on 14 Fr and larger tubes reduces the need for overdilation during both placement and replacement procedures.
- The durable balloon on the Entuit Thrive standard-profile feeding tube lasts 20.5 times longer than a leading tube providers standard-profile balloon.
- The feeding tube sizes match perfectly with the Entuit Initial Placement Gastrostomy Sets to streamline placement procedures.
- The medicine ports unique lid accommodates a 60 mL syringe and an oral syringe, to make feeding more convenient for patients.
 |  | 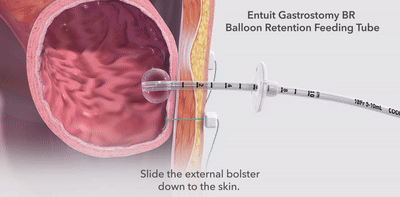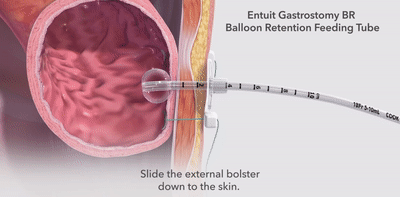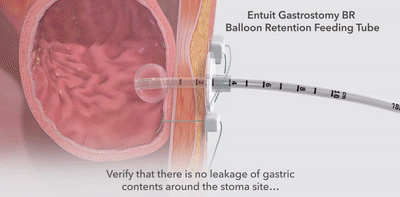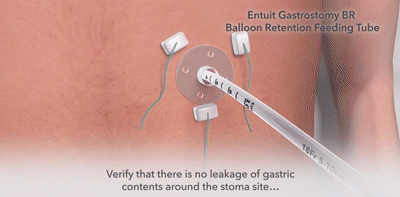 |
Intended Use
Intended to provide gastric access for enteral feeding, medication administration, and decompression through an established gastrointestinal stoma tract. Indicated for use in percutaneous placement of an enteral feeding tube in adult and pediatric patients that require enteral feeding, medication administration, or decompression through an established gastrointestinal stoma tract.
The Entuit Thrive Balloon Retention Gastrostomy Feeding Tube is intended to provide gastric access for enteral feeding, medication administration and decompression through an established gastrointestinal stoma tract.
The Entuit Thrive Balloon Retention Gastrostomy Feeding Tube is indicated for use in percutaneous placement of an enteral feeding tube in adult and pediatric patients that require enteral feeding, medication administration or decompression through an established gastrointestinal stoma tract.
Place The Feeding Tube with The Slim, Smooth, In-Line Balloon Shaft That Is Comfortable for Your Patient

Contraindications
Device placement is contraindicated for patients with evidence of granulation tissue, infection, and/or irritation in the stoma site.
Warning
- Inspect package integrity before use. Do not use device if package is damaged or if the sterile barrier has been compromised. Do not use if labeling is incomplete or illegible.
- Do not resterilize or reprocess this medical device as this may have an adverse effect on the known characteristics of the structural integrity, performance and biocompatibility of the device.
- For single patient use only. Do not reuse this medical device as this may increase the risk of contamination leading to transmission of infectious diseases which has the potential of resulting in patient injury, illness or death.
- FOR ENTERAL USE ONLY.The device is intended to connect to enteral giving sets for enteral feeding, and syringes for feeding, medication and inflation of balloon.
This device has KNOWN misconnections with connectors found in the following medical devices/healthcare applications:
- Intravascular devices;
- Hypodermic applications;
- Breathing systems and driving gas devices;
- Urethral/urinary devices;
- Limb cuff inflation devices;
- Neuraxial devices
Do NOT use this product in the vasculature.
Once used, dispose of packaging and package contents in accordance with healthcare institution guidelines and/or local government policy.
Precautions
This device should only be used for its intended purpose by or under the supervision of trained healthcare professionals with a comprehensive understanding in clinical principles, procedures and risks associated with percutaneous placement of enteral feeding devices. It is recommended to adhere to the instructions for use provided with this device, the enteral feeding instructions for the healthcare facility and instructions recommended by physicians.
Complications/Adverse Events
Complications and adverse events associated with placement and use of a balloon gastrostomy tube include, but are not limited to:
- Aspiration, reflux, sepsis, ascites, bleeding, peritonitis and perforation
- Granulation tissue, pressure necrosis and ulcers
- Irritation and infection such as redness, edema or purulent drainage
- Severe gastroesophageal reflux or diffuse inflammatory, infectious, or neoplastic disease involving the walls of the abdomen or anterior stomach
- Gastrointestinal obstruction and proximal small bowel fistulae
- Tube clogging, kinking, malposition,migration, leakage and unintended tube dislodgement.
How Supplied
Entuit Thrive Balloon Retention Gastrostomy Feeding Tube is provided sterile with one (1) Instructions for Use for each device.

