-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Baxter 2B0042 - 0.9% Sodium Chloride Injection, USP, 50 mL MINI-BAG Plus Container. Quad Pack, 80/CS
Baxter 2B0042 0.9% Sodium Chloride Injection, USP, 50 mL MINI-BAG Plus Container
0.9% Sodium Chloride Injection, USP in the MINI-BAG Plus Container is a sterile, nonpyrogenic solution for intravenous administration after admixture with a single dose powdered or liquid (up to 10 mL) drug vial. It contains no antimicrobial agents. The nominal pH is 5.0 (4.5 to 7.0). Each 100 mL contains 900 mg of Sodium Chloride, USP (NaCl). The osmolarity is 308 mOsmol/L (calculated). It contains 154 mEq/L sodium and 154 mEq/L chloride.
The MINI-BAG Plus Container is a standard diluent container with an integral drug vial adaptor. It allows for drug admixture after connection to a single dose powdered or liquid (up to 10 mL) drug vial having a 20 mm closure. A breakaway seal in the tube between the vial adaptor and the container is broken to allow transfer of the diluent into the vial and reconstitution of the drug. The MiniBag Plus product mechanically prohibits the transfer of contaminants into and out of the system during and after docking, minimizing environmental and personal exposure. The reconstituted drug is then transferred from the vial into the container diluent and mixed to result in an admixture for delivery to the patient.
The VIAFLEX Plastic Container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). Exposure to temperatures above 25 degree celcius/77 degree fahrenheit during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period. The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain chemical components from the plastic in very small amounts. However, biological testing was supportive of the safety of the plastic container materials.
Baxter 2B0042 Clinical Pharmacology
Sodium Chloride Injection, USP has value as a source of water and electrolytes. It is capable of inducing diuresis depending on the clinical condition of the patient.
Indications and Usage of Baxter 2B0042
0.9% Sodium Chloride Injection, USP is indicated as a source of water and electrolytes and may also be used as diluent for reconstitution of a powdered or liquid (up to 10 mL) drug product packaged in a vial with a 20 mm closure.
Directions for Use of Baxter 2B0042 Sodium Chloride Injection, USP, 50 mL MINI-BAG Plus Container
To Open
Tear overwrap down side at slit and remove solution container. Visually inspect the container. If the outlet port protector is damaged, detached, or not present, discard container as solution path sterility may be impaired. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
Prior to use, check that the vial adaptor cover is intact. Check the solution container for minute leaks by squeezing inner bag firmly. If leaks are found or if the vial adaptor cover is not intact, discard product as sterility may be impaired.
To Assemble and Reconstitute
See diagram for detailed instructions.
MINI-BAG Plus Container Directions Only For Single Dose Powdered or Liquid (up to 10 mL) Drug Vials with 20 mm Closures Use Aseptic Technique
Assembly
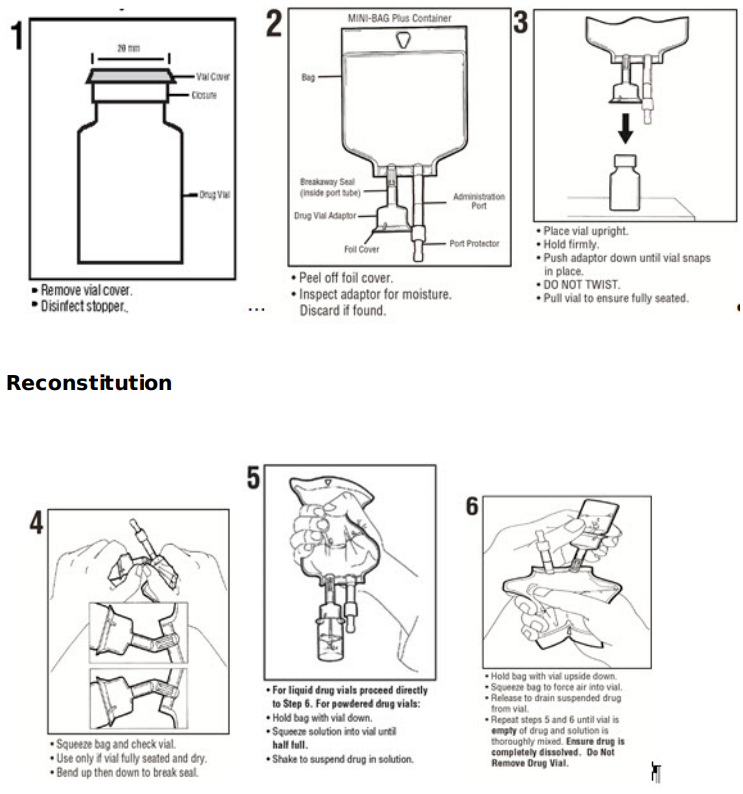
- Remove port protector. Attach administration set per its directions.
- Hang container on I.V. pole and prime set per directions. Ensure that vial is empty of drug and solution. Repeat step 6 if drug and solution remain in vial. Warning: Do not use in series connections.
- Administer medication per directions. Use within specified time for drug stability.

Baxter #2B0042, 0.9% Sodium Chloride Injection, USP, 50 mL MINI-BAG Plus Container. Quad Pack, 80/CS
$819.70 per CASE

Baxter #2B0040, Dextrose Injection, 5% USP, 50 ml, Mini-Bag Plus Container, Quad Pack, 80/cs (Rx) (Continental US Only, Excluding IN and ND)
$819.70 per CASE

