-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

3M 1492V - Indicator Biological Attest Super Rapid Readout Brown Cap 200/Ca

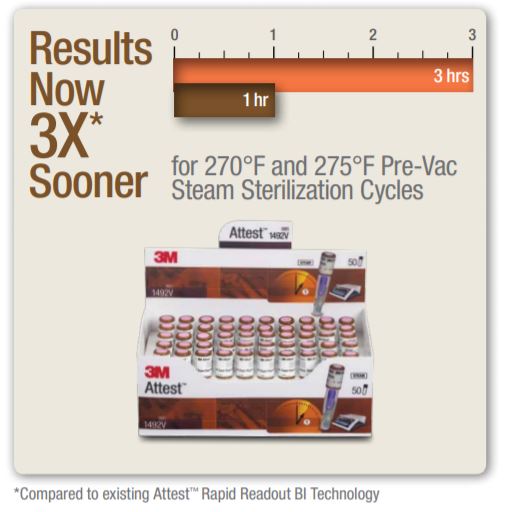
3M Attest Super Rapid Readout Biological Indicator For 132? (270?) 4 minute and 135? (275?) 3 minute Vacuum-Assisted Steam Sterilization cycles (Brown Cap)
The 3M Attest Super Rapid Readout Biological Indicator 1492V (brown cap, referred to hereinafter as the 1492V BI) is a self-contained biological indicator specifically designed for rapid and reliable qualification testing and routine monitoring of 270? (132?) and 275? (135?) dynamic-air-removal (pre-vacuum) steam sterilization processes when used in conjunction with the 3M Attest Auto-reader 490 (hereinafter referred to as the 490 Auto-reader). The 1492V BI is a single-use device.
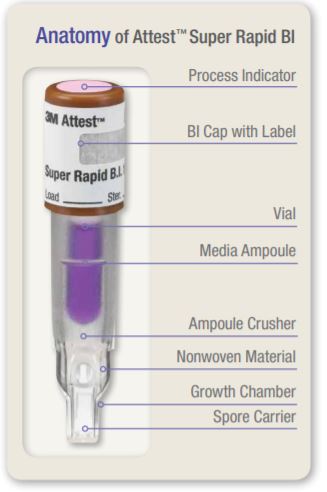
A schematic illustrating the design of the 1492V BI is provided in Figure 1. The self-contained design includes a carrier with spores of Geobacillus stearothermophilus and a media ampoule containing bacteriological growth medium which meets the requirements for growth promoting ability specified in ANSI/AAMI/ ISO 11138-1:2006/(R)2010. The spore carrier and media ampoule are contained in a plastic vial topped with a brown cap. A chemical process indicator which changes from pink to light brown or darker upon exposure to steam is located on the top of the cap.
- Provides BI results in 24 minutes in 3M Attest 490/490H Auto-readers with software version 4.2.7 or greater
- Dynamic-air-removal (Pre-vacuum and SFPP): 132? (270?) 3 or 4 min. 135? (275?) 3 min.
- Meets ISO and FDA performance requirements for biological indicators
- Contains viable organisms that provide a direct measure of lethality
- Puts your instrument turnaround speed into overdrive
- Puts your instrument turnaround speed into overdrive for improved patient safety
- *When used with 3M Attest Auto-reader 490 or the Attest Auto-reader 490H with software version 4.2.7 or greater.
The 1492V BI utilizes the a-glucosidase enzyme system, which is generated naturally within growing cells of Geobacillus stearothermophilus. The a-glucosidase in its active state is detected by measuring the fluorescence produced by the enzymatic hydrolysis of a non-fluorescent substrate, 4-methylumbelliferyl-a-D-gl ucoside (MUG). The resultant fluorescent by-product, 4-methylumbelliferone (MU), is detected in the 490 Auto-reader. The presence of fluorescence within 1 hour of incubation of the 1492V BI in the 490 Auto-reader indicates a steam sterilization process failure.
The 1492V BI can also indicate the presence of G. stearothermophilus organisms by a visual pH color change reaction. Biochemical activity of the G. stearothermophilus organism produces metabolic by-products that cause the media to change color from purple to yellow which also indicates a steam sterilization process failure. Use of this indication method is optional and is typically restricted to special studies.
Know Sooner.Because every minute matters.Get biological results in record time with Attest Super Rapid Readout Biological Indicators (BIs) and put your instrument turnaround speed into overdrive. The Attest Super Rapid Readout Advantage
| Know for Sure.Trusted technology, now optimized.3M Attest Super Rapid Readout Biological Indicators use the same technology youve trusted for years, now optimized to deliver an even faster result. Meets ISO and FDA requirements. Attest Super Rapid Readout BIs meet the performance requirements of ISO 11138-1:2006 and ISO 11138-3:2006, and satisfy FDA requirements for Biological Indicators.
A direct measure of lethality. Biological indicators (BIs) provide the only direct measure of the lethality of a sterilization cycle because they contain living, or viable, organisms. Each BI contains >1,000,000 living spores of a highly resistant organism. If the spores are inactivated or killed by the sterilization cycle, you have a direct measure of an effective sterilization process. No other sterilization monitoring device offers you this confidence. No added enzymes. Attest Super Rapid Readout BIs do not contain any added enzymes. The indicators use an enzyme present within the organism and generated during spore activation and outgrowth to provide you with Super Rapid results in an actionable time frame. |
Learn How A Faster Biological Indicator Result Can Help You...
...remain compliant with patient safety guidelines
- CDC, AORN & AAMI guidelines all state implants should be quarantined until a BI result is known, except in
emergency situations. With a faster BI result, the need for emergency release is reduced, which can lead to
improved patient safety. - A faster BI result can also result in less emergency release documentation.
...control costs
- Faster BI results can result in increased set turnaround and utilization, which can reduce the need to purchase
additional sets to have on hand. - Faster BI results can also reduce the need for additional loaner and consignment sets, reducing the indirect costs
associated with managing those sets.
...improve OR metrics
- When you have a faster BI result, turnaround speed can improve, which helps get implants and instruments to
- the OR faster. That speed can lead to better start-time accuracy, case duration rates, and room turnover rates.
...improve both surgeon and patient satisfaction
- When surgeons get what they need, when they need it, that responsiveness can lead to improved surgeon satisfaction.
- When properly sterilized implants get to the OR on time, surgeries are more likely to proceed without delay, which
- can lead to better patient outcomes and higher patient satisfaction.
...lower risk and severity of recalls
- Should a recall ever occur due to a sterilizer failure, having a faster BI result can help you respond faster, lessening
- the severity of the recall.
...improve YOUR peace of mind
- When implants are only released after the BI result is known, it brings peace of mind.
- When the OR gets what they need, when they need it, it brings peace of mind.
- When surveyor visits go smoothly because there are fewer emergency release forms on file, it brings peace of mind.
And when you know every patient under your watch is receiving the highest level of sterilization assurance possible, thats peace of mind

Readout Times
The 1-hour super rapid readout and the optional 48-hour visual pH color change incubation times have been correlated with a 7-day incubation period (at 56+/-2C) following the FDAs Reduced Incubation Time protocol. Processed indicators were examined at 48 hours and 7 days for detection of a visual pH color change. The 1-hour fluorescence change readings and the 48-hour visual pH color change readings were compared to the 7-day visual pH color change readings to determine the readout time of the indicator.
1-hour Fluorescence Change Result
1492V BIs have 1-hour reduced incubation time results that correlate to the 7-day (168 hours) visual readout result = 97% of the time.
48-hour Visual pH Color Change Result
1492V BIs have 48-hour reduced incubation time results that correlate to the 7-day (168 hours) visual readout result = 97% of the time.
Due to the high reliability of the 1-hour fluorescent result there is no advantage to incubating 1492V BIs beyond 1 hour. 1492V BIs meet ANSI/AAMI/ISO 11138-1:2006/(R)2010, ANSI/AAMI/ISO 11138-3:2006/(R)2010 and BS EN/ISO 11138-1:2006, BS EN/ISO 11138-3:2006.
Warnings
There is a glass ampoule inside the plastic vial of the biological indicator (BI). To avoid the risk of serious injury from flying debris due to a ruptured BI:
- Allow the BI to cool for the recommended time period before activating. Activating or excessive handling of the BI before cooling may cause the glass ampoule to burst.
- Wear safety glasses and gloves when removing the BI from the sterilizer.
- Wear safety glasses when activating the BI.
- Handle the BI by the cap when crushing or flicking.
- Do not use your fingers to crush the glass ampoule.
Precautions
1. DO NOT use the 1492V BI to monitor sterilization cycles which it is not designed to challenge:
- a. Gravity-displacement steam sterilization cycles;
- b. 250? (121?) dynamic-air-removal (pre-vacuum) steam sterilization cycles;
- c. Dry heat, chemical vapor, ethylene oxide or other low temperature sterilization processes.
2. After BI activation, ensure media has flowed to the spore growth chamber.
3. Do not place tape or labels on 1492V BI prior to sterilization or incubation in the 490 Auto-reader.
Monitoring Frequency
Follow facility Policies and Procedures which should specify a biological indicator monitoring frequency compliant with professional association recommended practices and/or national guidelines and standards. As a best practice and to provide optimal patient safety, 3M recommends that every steam sterilization load be monitored with a biological indicator in an appropriate Process Challenge Device (PCD i.e., BI challenge test pack).
Directions for Use
1. Identify the 1492VBI by writing the load number, sterilizer, and processing date on the indicator label. Do not place another label or indicator tape on the vial or on the cap.
2. Place the 1492VBI in a representative tray configuration or Process Challenge Device (PCD) as recommended by professional association guidelines or national standards for healthcare facility practice. Do not place the 1492VBI in direct contact with a chemical indicator as residue could transfer to the biological indicator and affect the result.
3. Place the PCD in the most challenging area of the sterilizer. This is typically on the bottom shelf, over the drain, however, the sterilizer manufacturer should be consulted to identify the area of the chamber least favorable to sterilization.
4. Process the load according to recommended practices.
5. After completion of the cycle take the PCD out of the sterilizer, and remove the 1492VBI.
6. Allow the 1492VBI to cool for 10 minutes prior to activation.
7. Check the process indicator on the top of the cap of the 1492VBI. A color change from pink to light brown or darker confirms that the 1492V BI has been exposed to the steam process. This color change does not indicate that the steam process was sufficient to achieve sterility. If the process indicator is unchanged, check the sterilizer physical monitors.
8. To activate the biological indicator, place it in a 490 Auto-reader incubation well which is color-coded brown (i.e., configured to incubate 1492VBIs). Press the cap of the BI down firmly to close the cap and crush the glass ampoule. Immediately remove the BI and flick it (see picture at right). Visually verify that media has flowed into the growth chamber at the bottom of the vial. If the media hasnt filled the growth chamber, hold the BI by the cap and flick it until media fills the growth chamber. Return the activated 1492V BI to the incubation well and wait for the result. See the 490 Auto-reader Operators Manual for further information related to its use.
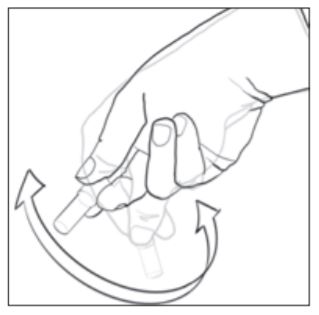
9. Each day that a processed 1492V BI is incubated, activate and incubate at least one non-processed 1492V BI to use as a positive control. Follow the activation instructions provided in Step 8 above. Write a "C" (for "control") and the date on the BI label. The positive control should be from the same lot code as the processed biological indicator. The positive control BI helps confirm:
- correct incubation temperatures are met,
- viability of spores has not been altered due to improper storage temperature, humidity or proximity to chemicals,
- capability of media to promote rapid growth, and
- proper functioning of the 490 Auto-reader.
10. Incubation and Reading: Incubate the positive control and steam processed 1492V BIs at 56 2C in a 490 Auto-reader. See the 490 Auto-reader Operator's Manual for the proper use of this equipment. Positive results are available within 1 hour. The 490 Auto-reader will indicate a positive result as soon as it is obtained. The final negative 1492V BI reading is made at 1 hour. After the results are displayed and recorded, the 1492V BIs may be discarded.
Interpretation of Results
Fluorescent Results
The positive control (unprocessed) 1492VBI must provide a positive fluorescent result (+ on the 490 Auto-reader LCD display). Processed 1492VBI results are not valid until the positive control reads fluorescent positive. The positive control should read positive (+ on the LCD display) at or before 1 hour. If the positive control reads negative (- on the LCD display) at 1 hour, check the 490 Auto-reader Operators Manual Troubleshooting Guide. Retest the 490 Auto-reader with a new positive control.
With processed 1492VBIs, a positive (+ on the LCD display) result indicates a sterilization process failure. A final negative processed 1492VBI reading (- on the LCD display) after 1 hour of incubation indicates an acceptable sterilization process. Act immediately on any positive results for processed BIs. Determine the cause of the positive BI following facility policies and procedures. Always retest the sterilizer and do not use sterilizer for processing loads until three consecutive BI results are negative.
Optional Visual pH Color Change Result
The 1492VBI is normally discarded after the fluorescent result has been recorded. If, however, special studies are desired, 1492V BIs may be further incubated for a visual pH color change result. After activation and during incubation, the white Nonwoven Material will absorb the bromocresol purple indicator, the pH-sensitive indicator dye in the growth media, and appear blue. In the case of the positive control BI a yellow color change of the growth media and/or Nonwoven Material will appear within 48 hours. Any observation of a yellow color within the vial indicates a positive result. In the case of a processed 1492VBI, a media and/or Nonwoven Material color change from purple to yellow indicates a sterilization process failure. A negative pH color change result, i.e., media and Nonwoven Material remain purple/blue, can be assessed at 48 hours.
Storage
- Best stored in original box under normal room conditions: 59-86? (15-30?), 35-60% relative humidity (RH).
- Do not store 1492VBIs near sterilants or other chemicals.
Disposal
Dispose of used 1492VBIs according to your health care facility policy. You may wish to steam sterilize any positive biological indicators at 132? (270?) for 4 minutes or at 275? (135?) for 3 minutes in a dynamic-air-removal steam sterilizer prior to disposal.

3M #1492V, Indicator Biological Attest Super Rapid Readout Brown Cap 200/Ca
$886.06 per CASE

3M #1291, Attest Biological Indicator Rapid Read 50/BX, 4 Bx Per/Cs, 200 Per/Cs
$599.00 per CASE

3M #1491, Attest Rapid Readout Sterilization Biological Indicator Vial Steam 2-1/2 Inch
$334.44 per BOX

3M #1291, Attest Biological Indicator Rapid Read 50/BX
Call for Pricing