By Category
-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
By Brand
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Part Number
3M 1660
SKU Number
CIA8038532
Sell Unit
CASE
Ships Within
Special Order
List Price
$1,125.20
Product Description
3M 1660 - Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing, 2 3/4" x 3 3/8", 25/bx, 4 bx/cs
3M 1660 Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing
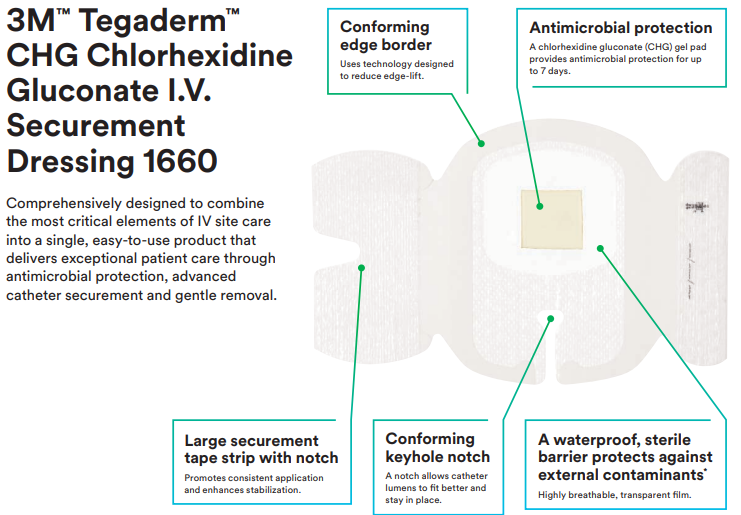
Comprehensively designed to combine the most critical elements of IV site care into a single, easy-to-use product that delivers exceptional patient care through antimicrobial protection, advanced catheter securement and gentle removal. The 3M Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing is an antimicrobial dressing that combines infection reduction, site visibility, consistent application and catheter securement in one integrated, easy-to-use product. It is the only transparent CHG dressing proven to reduce catheter-related bloodstream infections (CRBSI) and vascular catheter colonisation that aligns with evidence-based guidelines and practice standards.
Features and Benefits of 3M 1660 Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing
- Only transparent dressing cleared and proven to reduce catheter related bloodstream infections (CRBSI) and vascular catheter colonisation
- Integrated design combines infection reduction with site visibility, catheter securement and consistent application
- CHG gel pad provides immediate and continuous antimicrobial protection against microorganisms associated with CRBSIs for up to 7 days
- Transparent film and gel pad allows continuous site visibility to easily assess for early signs of infection
- Designed to minimise catheter movement and dislodgement
- Large securement tape strip with notch promotes consistent, correct application
- Can be worn up to 7 days
- Provides a waterproof, sterile barrier to external contaminants including liquids, bacteria and viruses.*
- Application frame makes placement accurate and easy, minimising potential to stick to gloves or to itself
- Application frame makes placement accurate and easy, minimizing potential to stick to gloves or to itself
How to Use 3M 1660 Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing
Prepare
- Prepare site according to facility protocol. Ensure site is completely dry.
Press
- Do not stretch the dressing at placement. Peel the liner from the dressing and place the CHG gel pad over insertion site (and suture sites when possible), aligning extension tubing with securement notch. Apply firm pressure to entire dressing with one hand for optimal adhesion, while removing border frame with opposite hand.
Secure
- Apply securement tape strip under the extension tubing and overlap back onto the dressing. Remove adhesive-free tabs.
- Document the dressing change information on the label strip. Apply label strip on top of dressing, over catheter lumen(s). Remove adhesive-free tabs. Secure tubing with tape.
Monitor
The dressing should be changed if the gel pad remains displaced when pressed with a finger. Change the dressing:
- At least every 7 days
- If the gel pad is saturated, when the dressing becomes loose or soiled, or in cases where there is swelling, visible drainage, or lost visibility
- If active bleeding or blood present outside the gel pad
Dressing is not intended to absorb large quantities of blood or drainage. Cover and protect dressing during patient bathing or showering.
Removal
Remove tape strips. Using a low and slow removal technique, start removing the dressing from where the catheter or tubing exits the dressing toward the catheter insertion site. Avoid skin trauma by peeling the dressing back rather than pulling it up from the skin. When the CHG gel pad is exposed, grasp a corner of the gel pad and dressing between thumb and forefinger. Apply a few drops of saline or alcohol if needed to facilitate removal of gel pad.
3M 1660 Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | Yes |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
;

