-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

3M 1837-2100 - KIT, PICC, SECURE DEVICE, TEGADERM DRESS, 80/CS

3M PICC / CVC Securement Device + Tegaderm I.V. Advanced Securement Dressing 1837-2100, 2 in x 2⅛ in. 5,1 cm x 5,4 cm (Overall Device Size), 3½ in x 4½ in 8,5 cm x 11,5 cm(Overall Dressing Size), 20 Device + Dressing/Box, 4 Boxes/Case
PICC / CVC Securement Device + Advanced Securement Dressing
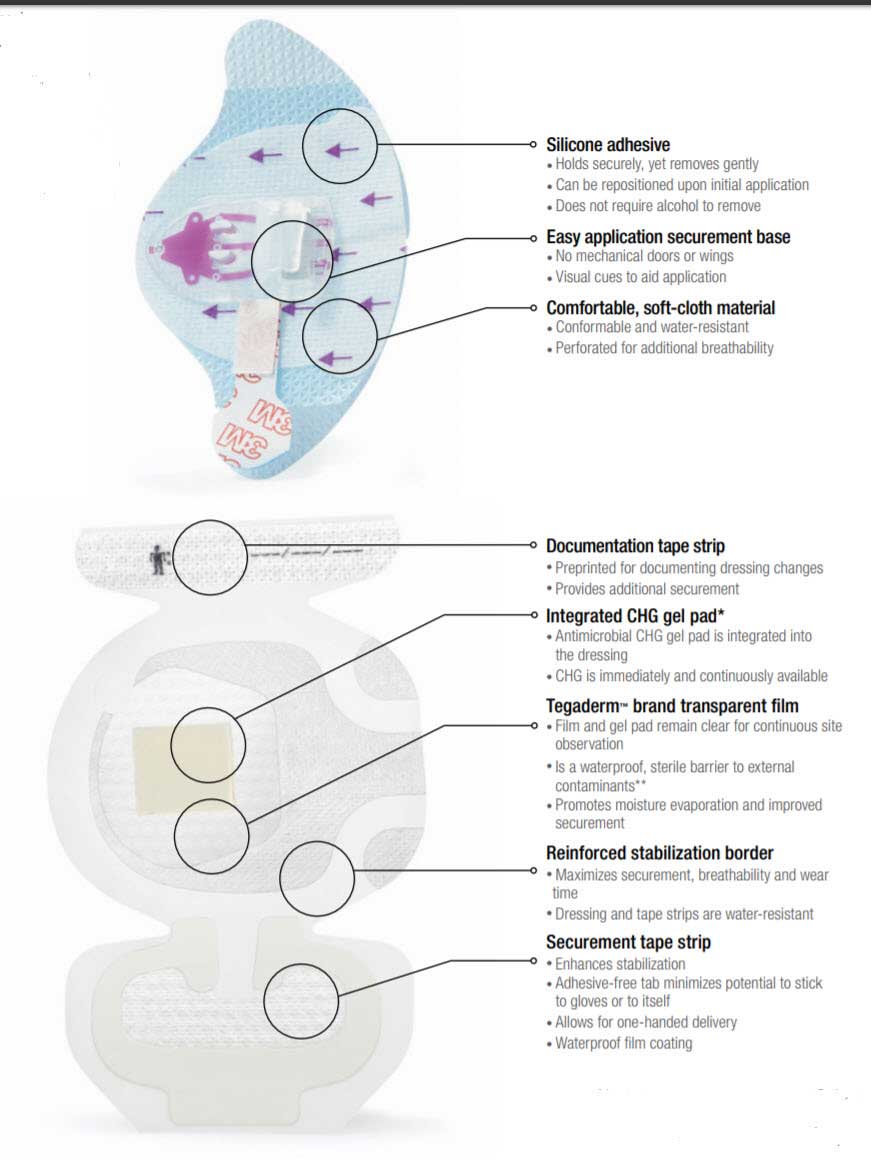
The Securement System consists of a device and a dressing. The molded plastic device is integrated onto a breathable base with a gentle silicone adhesive. The soft cloth bordered transparent film dressing is made of a thin film backing with a non-latex adhesive. A large, notched, film-covered soft cloth tape strip is included in the system. The Tegaderm I.V. Advanced dressing is breathable, allowing good moisture vapor exchange and has a transparent window, allowing continuous site observation. The transparent film provides an effective barrier against external contamination including fluids (waterproof), bacteria, viruses and protects the I.V. site.
- Sutureless securement balances reliable adhesion with gentleness to skin
- Removes cleanly, without causing patients undue pain or distress
- Accommodates the majority of PICC and CVC catheters up to and including 12 French
- Designed to be worn for up to 7 days
- Silicone adhesive holds securely, yet removes gently
- Eliminates the risks and costs of suture-related needlestick injuries
- Easy application securement base with no mechanical doors or wings
- A waterproof film coating provides a barrier to external contaminants including liquids, bacteria and viruses
Indications for Use
3M PICC/CVC Securement Device + Tegaderm I.V. Advanced Securement Dressing ("Securement System") is used to secure Peripherally Inserted Central Venous Catheters (PICCs) and Short Term Central Venous Catheters (CVCs) to the skin and to cover and protect catheter insertion sites. The device will accommodate the majority of single, double and triple lumen CVC catheters up to and including 12 French.
Reliable Securement Without Sacrificing Patient Comfort
Reliable securement of Peripherally Inserted Central Catheters (PICCs) and Central Venous Catheters (CVCs) is critical in avoiding the clinical, emotional and financial costs of complications. Traditional securement devices can be difficult and painful to apply and remove. Sutures can be uncomfortable for patients, and needlestick injuries (NSIs) can lead to significant burden on facilities and clinicians.
Building on over 30 years of experience collaborating with clinicians to simplify and improve I.V. site care, 3M designed securement systems that provide reliable securement without sacrificing the comfort patients deserve.
Balances Reliable Adhesion with Gentleness to Skin
- Secures as well as, or better than, leading securement devices and sutures
- Designed to be worn for up to 7 days
- Removes gently, without causing patients undue pain or distress
- As comfortable as, or more comfortable than, leading securement devices and sutures
- Improves patient comfort, mobility and satisfaction
Makes Your Life Easier
- Easier to apply and remove than leading securement devices
- Accommodates the majority of PICC and short-term CVC catheters up to, and including, 12 French
- To optimize placement, device can be repositioned upon initial application without losing adhesion
- Single package, with device and dressing simplifies product selection
- Supports OSHA and CDC guidelines for sutureless securement
Reduces Overall Cost of Care
- Eliminates the risks and costs of suture-related needlestick injuries
- Can potentially reduce the number of dressing changes and restarts
- Combined system means fewer products to purchase and stock
- Seven-day wear time could reduce costs for unscheduled visits due to securement or dressing failure
Provides Antimicrobial Protection
- Integrated CHG gel pad surrounds insertion site in CHG, including under the catheter hub
- Kills and suppresses regrowth of pathogens on the skin and catheter
- CHG is immediately active, and continuously available for up to 7 days
Designed with Clinicians and Patients In Mind
The 3M PICC/CVC Securement Systems were specifically designed to provide:
- Secure adhesion
- Gentle removal
- Antimicrobial protection
- Site visibility
- Long wear time
- Easy application and removal
- Patient comfort and mobility
Gentle on Skin for Improved Comfort
The device removes cleanly, eliminating the need to scrub with alcohol. Removal of other stabilization devices can cause adhesive trauma, stripping skin cells along with the device. Significantly fewer skin cells are removed when removing the 3M PICC/CVC Securement Device than the Bard StatLock Tricot stabilization device, proving that it is more gentle to skin.
Easy to Apply and Remove
The 3M PICC/CVC Securement Systems were designed for easier application and removal. An evaluation comparing the 3M PICC/CVC Securement System to the Bard StatLock Stabilization Device for Peripherally Inserted Central Catheters (PICCs), showed the 3M PICC/CVC Securement System to:
- Be easier to apply and remove
- Be easier to remove without catheter movement
- Leave minimal to no adhesive residue on skin upon removal
- Be repositionable upon initial application without losing adhesion
Reduces the Risks of Suture-Related Needlestick Injuries
The 3M PICC/CVC Securement Systems are an ideal alternative to sutures, helping to eliminate the unnecessary financial, physical and emotional costs of suture-related needlestick injuries (NSIs). Health care workers in hospitals experience approximately 92,400 suturerelated sharps or NSIs each year. NSIs expose workers to bloodborne pathogens including HIV, Hepatitis B, Hepatitis C, and others, and can pose significant burdens, including:
- Trauma-related psychiatric disorders
- Loss of employee time
- Cost of staff to investigate the injury
- Expense of laboratory testing
- Cost of treatment for infected staff
- Cost of replacing staff
The Centers for Disease Control and Preventions 2011 Guidelines for the Prevention of Intravascular Catheter-Related Infections recommend the use of a sutureless securement device to reduce the risk of infection for intravascular catheters.
Proven to Provide Reliable PICC/CVC Securement
Peripherally Inserted Central Catheters (PICCs)
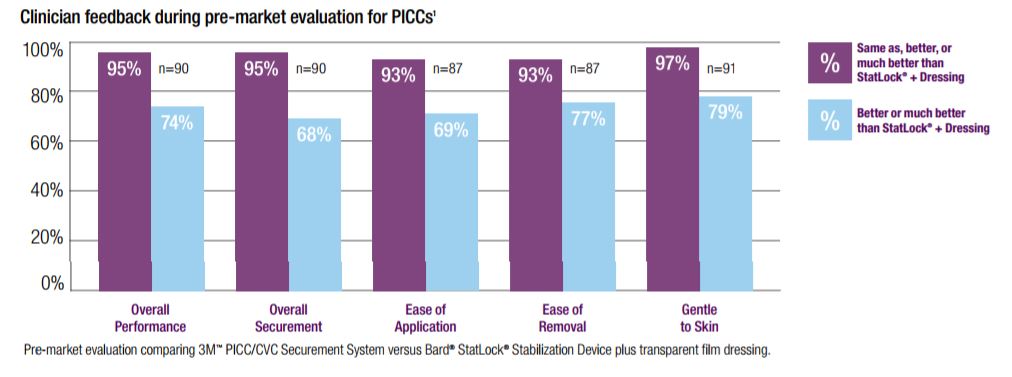
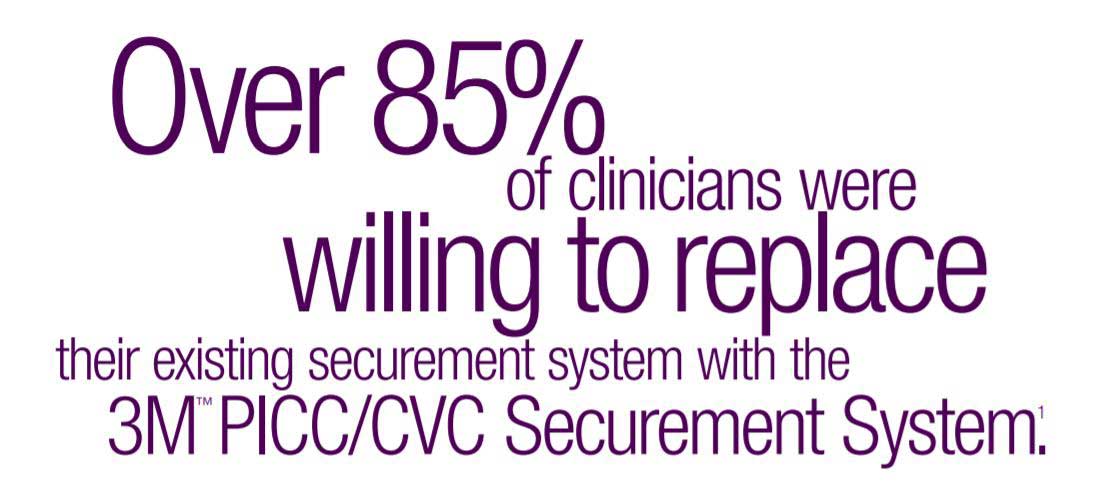
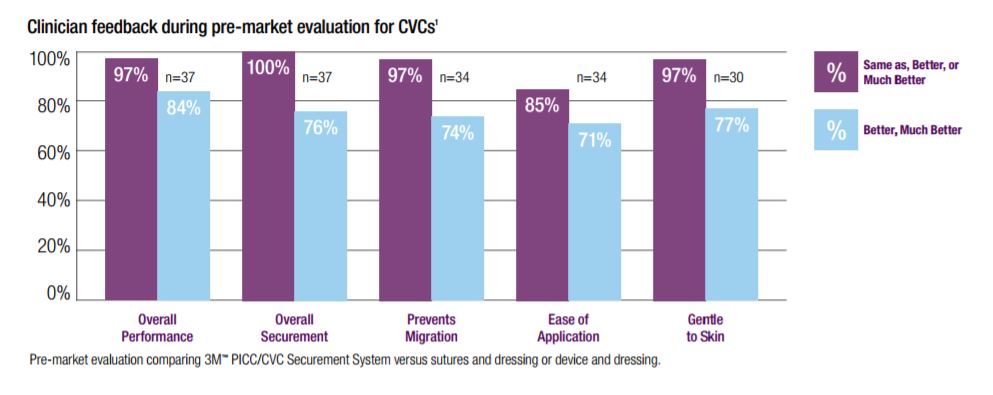
The 3M PICC/CVC Securement Systems were designed to minimize catheter migration or dislodgement complications. In a pre-market evaluation, clinicians rated the 3M PICC/CVC Securement System as providing better overall PICC securement than the Bard StatLock Stabilization Device and transparent film dressing. The 3M PICC/CVC Securement System was also rated to be easier to apply and remove and gentler to skin than StatLock. In fact, 85% of the clinicians indicated they would be willing to replace their existing PICC securement system with the 3M PICC/CVC Securement System.
In a simulated clinical situation, the 3M PICC/CVC Securement System could withstand the sudden, high pull force of dropping an attached 2.5 pound weight 100% of the time, while the StatLock PICC Plus with a Tegaderm I.V. Film Dressing with Border (1655) and a Competitive Securement Dressing failed every time.
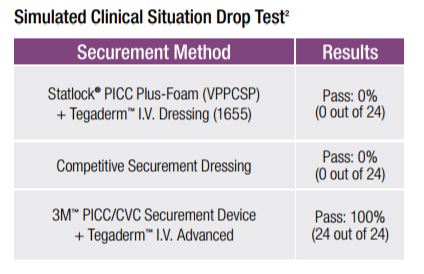
3M PICC/CVC Securement System could withstand a sudden, high pull force of an attached 2.5 pound weight being dropped 24 out of 24 times.
Central Venous Catheters (CVCs)
In a pre-market evaluation, clinicians rated the 3M PICC/CVC Securement System as providing better overall securement than sutures and a dressing or a securement device and dressing. The 3M PICC/CVC Securement System was also rated higher in preventing migration, ease of application and gentleness to skin. In fact, 90% of the clinicians indicated they would be willing to replace their existing CVC securement system with the 3M PICC/CVC Securement System.
In vivo testing comparing the mean pull force required to dislodge an inserted CVC catheter with various securement devices, showed the 3M PICC/CVC Securement System could withstand significantly higher pull force than a Competitive Securement Dressing or sutures.

3M PICC/CVC Securement Systems require higher pull force than sutures and dressing or Competitive Securement Dressing, and more than 1.5 times more than sutures (3-0 silk) plus Tegaderm I.V. Film Dressing with Border (1655).
Application & Removal Guide
Suggested Applications
- Peripherally Inserted Central Catheters (PICCs)
- Short-term Central Venous Catheters (CVCs)
Securement System Selection
Choose the system with a dressing large enough to provide at least a one-inch margin of adherence on dry, healthy skin around the catheter site.
Site Preparation
- Prepare the site according to your facilitys protocol.
- Clipping hair at the site may improve dressing adhesion. Shaving is not recommended.
- 3M Cavilon No Sting Barrier Film can be used with the PICC/CVC dressing. Apply Cavilon No Sting Barrier Film evenly to the skin. Avoid the area immediately surrounding the insertion site.
- Allow all preps and protectants to dry completely before applying the securement device and Tegaderm I.V. Advanced Dressing to ensure good adhesion and prevent skin irritation.
Site Care
- Follow universal precautions as indicated by established protocols for infection prevention.
- Observe site daily for signs of infection or other complications. Infection may be signaled by fever, pain, redness, swelling, or unusual odor or discharge.
- If infection is suspected, remove the Tegaderm I.V. Advanced Dressing, inspect the catheter site directly, and determine appropriate medical intervention.
- Inspect the 3M PICC/CVC Securement System daily and change the system as necessary, in accordance with facility protocol.
- Securement System changes should occur at least every 7 days and may be needed more frequently with highly exudative sites or if integrity of the device or dressing are compromised.
Reducing Infection Risks
- At each securement system change, pay careful attention to disinfecting the skin around and under the catheter.
- Carefully disinfect injection ports before access.
- Inspect the site frequently for early signs of complications. Transparent dressings allow easy site assessment without removing the dressing.
- Change the securement system according to your facilitys protocol, or when it becomes wet, loosened or soiled. Edge lift of a transparent dressing is not necessarily failure, unless there is a channel from the edge of the dressing to the insertion site of the I.V. catheter.
- Use maximum barrier precautions for central venous catheter (CVC) insertion and sterile technique for site care.
- Protect against skin irritation. Compromised skin near the catheter insertion site increases the risk of complications.
- No dressing can substitute for your professional site care.
Precautions
- Stop any bleeding at the site before applying the securement system.
- Do not stretch the dressing during application as tension can cause skin trauma.
- Make sure the skin is clean and free of soap residue and lotion. Allow skin to dry thoroughly before applying the securement system to prevent skin irritation and to ensure good adhesion.
- The securement system may be used on an infected site, only when under the care of a health care professional.
- Antimicrobial ointments containing polyethylene glycols may compromise the strength of the securement system.
- Tegaderm I.V. Advanced Dressings should not be re-sterilized by gamma, E-beam or steam methods.


Specifications

3M PICC/ CVC Securement System - Frequently Asked Questions
What is the 3M PICC/CVC Securement System made of?
The 3M Tegaderm I.V. Advanced Securement Dressing features a pattern-coated adhesive on a highly breathable film. The waterproof film covers the dressing and stabilization securement tape strips. The securement device is formed from polycarbonate resin material. The silicone adhesive of the securement device provides strong yet gentle adhesion to the skin.
Do I have to use the dressing provided with the 3M Securement Device or can I use it with another dressing?
Yes, the Tegaderm I.V. Advanced Securement Dressing included in the system was specifically designed to enhance securement of the device, ensuring the best possible experience and outcomes.
What kind of intravascular catheters will this securement system secure and fit properly?
The 3M PICC/CVC Securement System will secure single, double and triple lumen PICCs and short-term CVCs up to and including 12 French. The securement device does not fit well on catheters without a "winged" hub, like most arterial catheters or those with a luer-lock type connection.
What do the CDC guidelines recommend for PICC or short-term CVC securement?
Catheter stabilization is recognized as an intervention to decrease the risk for phlebitis and catheter migration and dislodgement, and may be advantageous in preventing CRBSIs. Pathogenesis of CRBSI occurs via migration of skin flora through the percutaneous entry site. Sutureless securement devices avoid disruption around the catheter entry site and may decrease the degree of bacterial colonization (Yamamoto, et al, 2002). Using a sutureless securement device also mitigates the risk of sharps injury to the health care provider from inadvertent needle stick injury.
What is the definition of a short-term CVC?
A short-term CVC is classified by the FDA in 21 CFR, Section 880.5200 Intravascular catheter: "An intravascular catheter is a device that consists of a slender tube and any necessary connecting fittings that is inserted into the patient's vascular system for short-term use (less than 30 days) to sample blood, monitor blood pressure, or administer fluids intravenouslyThe device may be constructed of metal, rubber, plastic, or a combination of these materials."
What is the definition of a peripherally inserted central catheter (PICC)?
A CVC with placement access via a peripheral vein.
When should the dressing or device be changed?
The Securement System should be changed at least every seven days and may need to be changed more frequently with highly exudative sites or if integrity of the dressing is compromised. The securement device should be changed with each dressing change. Change the dressing when it becomes damp, loosened, or visibly soiled (CDC, 2011).
How is this securement system classified by the FDA?
The non-CHG dressing is classified by FDA in 21 CFR, Section 878.4020 Occlusive wound dressing as a Class I device which is exempt from Premarket Notification (510k). The securement device is classified by FDA in 21 CFR, Section 880.5210 Intravascular catheter securement device as a Class I device which is exempt from Premarket Notification (510k).
Is the silicone adhesive the same used in 3M Kind Removal Silicone Tape?
This silicone adhesive provides stronger adhesion for securing critical tubing than the silicone adhesive found in 3MKind Removal Silicone Tape, while still removing gently.
Will this system be available with Tegaderm CHG I.V. Securement Dressing?
The 3M PICC/CVC Securement Device + Tegaderm CHG Chlorhexidine Gluconate I.V. Securement Dressing is currently undergoing premarket review (pending 510k) by the FDA to be used to secure PICCs and short-term CVCs to the skin and cover and protect catheter insertion sites. The proposed intended use of the device is to accommodate the majority of single, double and triple lumen CVC catheters up to and including 12 French.
Is this system compatible with other antimicrobial dressings or sponges?
Yes.
Can this system be used in combination with sutures?
The 3M PICC/CVC Securement System was designed to replace sutures. The 2011 CDC Guidelines recommends the use of a sutureless securement device to reduce the risk of infection for intravascular catheters (Yamamoto, et al, 2002).
Can the dressing be used if the site is bleeding or oozing?
If the site is bleeding or oozing, use a gauze dressing until this has resolved (CDC, 2011). Refer to facility protocol.
Does the 3M PICC/CVC Securement System fit with an Arrow Box Clamp?
Yes, the securement system will fit with a catheter that has an Arrow Box Clamp. Secure any additional catheter length per facility protocol.
Can the Securement System be used with a variety of antimicrobial skin preps and skin protectants?
Yes, however, antimicrobial ointments containing polyethylene glycols may compromise the film strength of the Tegaderm I.V. Advanced Dressing. Testing indicates the 3M Securement System is safe to use with antiseptic preps:70% Isopropyl Alcohol, Povidone Iodine, and ChloraPrep-2% CHG (3M data on file). Testing indicates the Securement System is safe to use with 3M Cavilon No Sting Barrier Film, a skin protectant that is chemically compatible with CHG and provides superior skin protection to adhesive trauma compared to other skin protectants.
What clinical information and studies have been performed on this product?
Pre-market evaluation has been conducted; included over 20 hospitals and 130 evaluators. Additional evidence is available on the 3M Securement System website (3M.com/Securement) and in the product brochure, which includes 3M internal clinical studies, such as a 2.5 pound drop test and peak axial pull force testing.
Please explain the repositionable attributes of the securement device.
Based on 3M internal clinical studies, the securement device may be repositioned at the time of initial application without a reduction in securement.
Is the 3M PICC/CVC Securement System MRI-compatible?
Yes, the Securement System is compatible with MRI. This product does not contain metal or conductive materials, which have been found unsafe for use in MRI.

3M #1837-2100, KIT, PICC, SECURE DEVICE, TEGADERM DRESS, 80/CS
$825.12 per CASE

3M #1837-2100, KIT, PICC, SECURE DEVICE, TEGADERM DRESS, 20/BOX
$229.79 per CASE

3M #1837-2100, KIT, PICC, SECURE DEVICE, TEGADERM DRESS, EACH
$19.00 per EACH

3M #1839-2100, KIT, PICC, SECUR DEVICE, TEGADRM DRESS, L, 80/CS
$731.89 per CASE

