-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

3M 90601 - DRESSING, FOAM, TEGADERM, NON-ADH, 4"X4", 10/BX
3M Tegaderm Foam High Performance Foam Non-Adhesive Dressing, 4 in x 4 in (10cm x 10cm), 10 Dressings/Box, 4 Boxes/Case
Absorbent Breathable & Conformable
3M Tegaderm High Performance Foam Non-Adhesive Dressing is a highly absorbent, breathable, non-adherent wound dressing. It is constructed from a conformable polyurethane foam pad, absorbent nonwoven layers, and a top layer of printed adhesive film. This waterproof film is moisture vapor permeable which prevents wound exudate strike-through and acts as a barrier to outside contamination, including bacteria and viruses.* The dressing maintains a moist wound environment, which has been shown to enhance wound healing.
A non-adhesive foam dressing that effectively handles low to high exudating wounds. Innovative layer technology absorbs and evaporates moisture to maintain an optimal wound healing environment.
Absorbs & Evaporates
Innovative layer technology absorbs and evaporates moisture to maintain an optimal wound healing environment.
- Innovative fluid handling technology
- Unique multi-layer design provides high absorbency with breathability to reduce the risk of maceration
- Adapts to changing levels of exudate to maintain moisture balance for optimal wound healing
- Effective under compression
- Easy to use
3M Tegaderm High Performance Foam Non-Adhesive Dressing
3M Tegaderm High Performance Foam Non-Adhesive Dressing is a highly absorbent, breathable, non-adherent wound dressing. It is constructed from a conformable polyurethane foam pad, absorbent nonwoven layers, and a top layer of printed adhesive film. This waterproof film is moisture vapor permeable which prevents wound exudate strike-through and acts as a barrier to outside contamination, including bacteria and viruses.* The dressing maintains a moist wound environment, which has been shown to enhance wound healing.
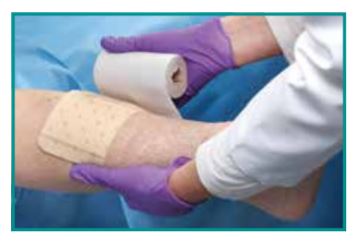

Indications for Use
Tegaderm Foam Dressing is indicated for use as a primary or secondary dressing for low- to highly-exuding partial and full thickness wounds such as pressure ulcers, venous leg ulcers, neuropathic ulcers, arterial ulcers, skin tears, surgical wounds, abrasions, superficial partial thickness burns and donor sites. The dressing is suitable for use around tube exit sites and with compression therapy. The dressing is not designed, sold or intended for use except as indicated. Tegaderm Foam Dressing is not intended for use in pressure reduction or as a surgical sponge.
- Pressure ulcers
- Ulcers of the lower extremity including; venous leg ulcers, arterial ulcers and neuropathic (diabetic) ulcers
- Skin tears
- Donor sites
- Surgical wounds
- Abrasions
- Superficial and partial thickness burns
- Suitable for use around tube exit sites
- Suitable for use with compression therapy
Follow Universal Precautions and Facility Guidelines for Infection Control
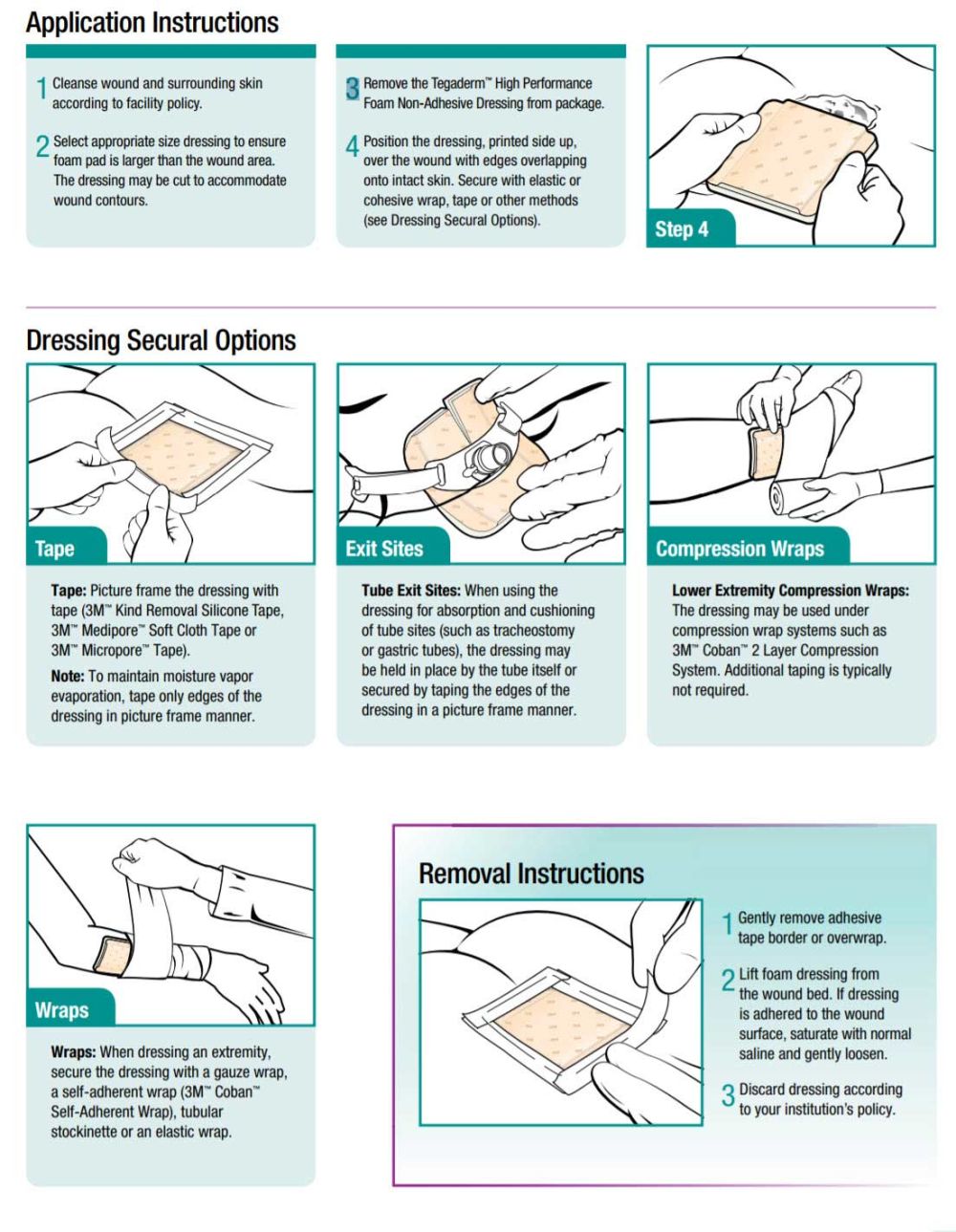
3M Tegaderm High Performance Foam Non-Adhesive Dressing
Considerations
Observe the dressing frequently. As the dressing absorbs, the exudate will wick to the top of the dressing, and discoloration may be noticeable. When the exudate spreads to the edges of the dressing or the dressing begins to leak, a dressing change is indicated.
- Tegaderm Foam Dressing may remain in place for up to seven days. Dressing change frequency will depend on the type of wound, volume of exudate, and clinical situation. Change at least every seven days, or as indicated per treatment protocol.
- The dressing may be used as an absorbent cover dressing over wound fillers such as alginates or gauze packing.
- The dressing may be cut as necessary to accommodate wound size and contours. Use of non-sterile product may increase the risk of contamination and infection.
- Covering the entire foam dressing with an occlusive tape may reduce the breathability of the film backing. To maintain moisture vapor exchange, tape only edges of the dressing in picture frame manner.
Suggested Applications
- Pressure, arterial, neuropathic (diabetic) and venous leg ulcers, including use under compression wraps
- Skin tears and abrasions
- Donor sites
- Superficial partial thickness burns
3M Tegaderm Foam Dressing (nonadhesive) | Commonly Asked Questions
Question: What is the best method to secure Tegaderm Dressing (nonadhesive)?
Answer: Tegaderm Foam Dressing (nonadhesive) may be secured by any secondary method the clinician prefers: Adhesiveless options:
roll gauze
- elastic or cohesive wraps, e.g., 3M Coban Self-Adherent Wrap (applied without tension)
- tubular netting or stockinette.
Adhesive options:
- 3M Medipore H Soft Cloth Surgical Tape, conformable and gentle
- 3M Transpore White Dressing Tape, conformable and gentle
- 3M Tegaderm Transparent Dressings if contamination may occur
(i.e., protection from incontinence); or the clinician may wish to consider
3M Tegaderm Foam Adhesive Dressings for these applications.
Question: Can Tegaderm Foam Dressings be used over infected wounds?
Answer: Yes. Tegaderm Foam Dressings may be used on infected wounds only under the care of a health care professional. See package insert for additional information.
Question: Is there a correct way to apply Tegaderm Foam Dressing (nonadhesive)?
Answer: Yes. Tegaderm Foam Dressing (nonadhesive) is designed with a white, nonadherent wound contact side and an outer, barrier film side with a printed blue grid and 3M logo. (Remember "Blue side - Sky Up!") 3M 11/28/06 Page 2 of 3 106002QA.pdf
Question: Can the blue, printed grid be used as a measuring device?
Answer: No. Because the wound cannot be seen through the dressing, measurements cannot be obtained. However, the grid may be useful (for the clinician establishing directions/protocols) for determining when the dressing needs to be changed and as a reminder of how to apply the dressing correctly. For example, when exudate is seen near the outer part of the grid, it is time to change the dressing.
Question: Can Tegaderm Foam Dressings be used for hypergranulation tissue?
Answer: Yes. Hypergranulation tissue refers to an exuberant, over-development of new blood vessel tissue in a chronic wound (you may hear it described as "proud flesh"). The etiology of this condition is not clearly understood but anecdotally has been associated with several conditions including over-hydration of the wound bed with the use of occlusive dressings and a prolonged inflammatory phase due to a wound surface infection or foreign body debris. Tegaderm Foam Dressings are useful for managing these wounds because of their higher TOTAL FLUID MANAGEMENT. In addition, they leave no residue in the wound that could potentially prolong inflammation. Some clinicians believe that foam dressings also provide a beneficial, light topical pressure to the wound surface, especially when used with compression dressings, which acts to inhibit the overgrowth of granulation tissue.
Question: Do Tegaderm Foam Dressings provide barrier protection against contamination, bacteria and viruses?
Answer: Tegaderm Foam Dressing (nonadhesive) is covered with a highly breathable film* backing which prevents wound exudate strike-through and acts as a barrier to outside contamination. The edges of the dressing must be bordered with 3M Tegaderm Transparent Dressing for barrier protection against bacteria and viruses. 3M Tegaderm Foam Adhesive Dressings are constructed with a top layer of transparent adhesive film which is moisture vapor permeable, prevents wound exudate strike-through and acts as a barrier to outside contamination, including bacteria and viruses*. *Laboratory testing has proven that the film provides a barrier against HIV-1 and HBV while the dressings remain intact without leakage.
Specifications

3M #90601, DRESSING, FOAM, TEGADERM, NON-ADH, 4"X4", EACH
$5.40 per EACH

3M #90601, DRESSING, FOAM, TEGADERM, NON-ADH, 4"X4", 40 EA/CS, 4 BX/CS
$356.94 per CASE

3M #90601, DRESSING, TEGADERM FOAM 4"X4" (10/BX)
$74.25 per BOX

3M #90601, DRESSING TEGAFOAM NONADHESIVE 4X4 10/BX 4BX/CA
$164.74 per CASE


