-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

3M 90614 - Tegaderm Foam Dressing 2.75x3 10/Bx, 4 BX/CA
3M Tegaderm High Performance Foam Adhesive Dressing 90613, Mini Wrap, 2.75 in. x 2.75 in. 6,9 cm x 6,9 cm
Wound Management Simplified
Tegaderm Adhesive Foam Dressings can help simplify decision ma king, increase consistency of care, manage costs and improve outcomes. Helps meet the challenges for low to high-exudating wounds.
- Intuitively adjusts to changing levels of exudate
- Highest fluid handling capacity among leading adhesive foam brands*
- Adheres to both dry and moist skin
- Reduces the time and costs of more frequent dressing changes
Features
- Unique multi-layer design provides high absorbency with high breathability to reduce the risk of maceration
- Adapts to changing levels of exudate to maintain moisture balance for optimal wound healing
- Ideal for situations where increased securement or longer wear time is needed
- Conformable for difficult body contours
- Dressing does not stick to wound bed thereby minimizing disruption of healing tissue
- Provides a water resistant seal while dressing is intact.
- Spoke delivery provides easy application
- Film backing prevents strikethrough and protects against external bacteria and viruses* *In vitro testing shows that the transparent film provides a viral barrier from viruses 27 nm in diameter or larger while the dressing remains intact without leakage.
2-3/4 inch x 2-3/4 inch (7cm x 7cm) Foam Adhesive Dressing, Mini Wrap, Pad size 1 inch round x 1 inch round (2,5cm x 2,5cm).
An adhesive foam dressing that effectively handles low to moderate exudating wounds. Innovative layer technology absorbs and evaporates moisture to maintain an optimal wound healing environment. For easy application to difficult body contours such as fingers and toes.
Suggested Applications
- Pressure, arterial, neuropathic (diabetic) and venous leg ulcers
- Difficult body contours
- Skin tears and abrasions
- Superficial partial thickness burns
The Complexity of Chronic Wound Care
The burden of chronic wound care on patients, clinicians and health care systems is significant. The aging population, rising incidence of obesity, prevalence of diseases such as diabetes, and other factors contribute to the rising number of chronic wounds, and the associated costs for treating them.
- 7 million people will suffer from chronic wounds this year.
- There has been a 132% increase in obesity in the past 20 years.
- 8.3% of the population has diabetes.
- More than 2.5 million people develop pressure ulcers each year.
Chronic wounds can be disabling and constitute a significant burden on patients activities of daily living. Wound care decision making can be complex, requiring clinicians to adapt dressing selection and procedures to accommodate varying levels of exudate and difficult body contours. Advanced wound care products are helping to bring more science to the art of managing wounds, but the multitude and complexity of options can further complicate decision making.
Managing More with Less
The ultimate challenge for health care providers is managing more patients with fewer resources due to limited staff, overloaded work schedules, and high turnover. Changes in reimbursement policies have exerted even more pressure on providers to improve outcomes while cutting costs. Increasingly, facilities are trying to standardize on products that can be used across the continuum of care to ensure consistency, save money and provide better total patient care.
The Brand You Trust To Simplify Wound Management
We can help you simplify decision making, increase consistency of care, manage costs and improve outcomes with formulary and protocol development that include Tegaderm Adhesive Foam Dressings, consulting, training, and clinical support.
The Ideal Dressing for the Majority of Wounds
Tegaderm Adhesive Foam Dressings intuitively adjust to changing levels of exudate to maintain an optimal moisture balance and reduce the risk of maceration, so you can feel confident youre providing the best care possible for almost any wound.
Reliability You Can Count On
You have enough on your plate. Tegaderm Adhesive Foam Dressings are easy to apply and easy to teach. They have the highest fluid handling capacity among leading adhesive foam brands*, so they're less likely to fail prematurely due to oversaturation. The dressings adhere to both dry and moist skin, even on challenging body parts. The highly breathable film protects the wound, maintains an optimal healing environment and prevents strikethrough. This means you can use your limited time focusing on other areas of care.
Helping you Manage Costs
You can reduce the time and product costs incurred performing more frequent, or unscheduled, dressing changes making Tegaderm Adhesive Foam Dressings the cost-effective choice for the majority of wounds.
Improving Patient Activities of Daily Living
You can impact the health and well-being of your patients by providing treatment that enables them to live their lives more comfortably. The foam pad doesnt adhere to the wound bed, protects periwound tissue, and minimizes pain. The conformable, low-profile dressing can be worn with normal clothing and footwear, and the waterproof seal enables patient showering.
Case-Series - Use of a Foam Adhesive Dressing? on Chronic Wounds
Case Series Summary
Background: Case series studies are an important element in understanding the clinical use of dressings on a variety of wounds to which they are normally exposed. In this study an adhesive foam dressing with a demonstrated high in vitro MVTR and a high in vivo fluid handling capacity was studied on a variety of wound types and in a variety of clinical settings.
Methods: The wounds selected were low to high exuding, partial and full thickness dermal wounds that were currently being treated with a foam dressing and were expected to use the study foam dressing for four weeks. Weekly dressing changes were recommended if possible, but more frequent changes were not restricted. A total of 24 patients were enrolled into the study.
Baseline Demographics:
- Facility: 29% Home Care, 33% Long-Term Care, 38% Wound Clinic
- Gender: 54% Female, 46% Male
- Mean age = 68.2 (19-91) Years
- Mean weight = 212.1 (102-596) lbs.
- Wound Type: 50% Pressure, 17% Venous, 29% Surgical, 4% Trauma
- Wound Location: 38% Sacrum/Coccyx/Buttock, 21% Foot/Heel, 17% Abdomen, 17% Lower Leg/Knee, 8% Other (Flank/Spine)
- Wide range of wound age and pre-study foam use
Overall Dressing Assessments:
- 72% of the 140 dressing applications were rated as having good or very good Ease of Application
- Of the 116 dressing changes, 76% were rated as having good or very good Ease of Removal
- 73% rated the dressing as very comfortable or comfortable
- 66% rated the dressing as maintaining a moist wound environment
- 89% of dressing changes were due to routine care, 3% due to leakage, 3% due to soiled dressing
- Wear Time: Mean (SD) = 4.8 (2.4) days (n=115 dressing applications) with the investigators stating that 51% of the dressings could have remained on longer, and at the end of the study they indicated that for all 24 subjects the dressing met their wear time expectations.
Baseline Wound Area and Change:
- Nine out of the 24 (38%) subjects had their wounds heal during the study.
- Baseline wound area ranged from 0.29 cm2 to 111.6 cm2 with a mean of 10.92 cm2 (median 3.9 cm2 ); At last visit the mean area was 6.4 cm2 (median 0.17 cm2 ) with a mean area reduction of 70.8% (median 93.2%) and a mean absolute area reduction of 4.5 cm2 (median 2.08 cm2 ). The last visit occurred between 5 and 35 days of the first dressing application (mean of 22.4 days and median 23 days of test dressing use.
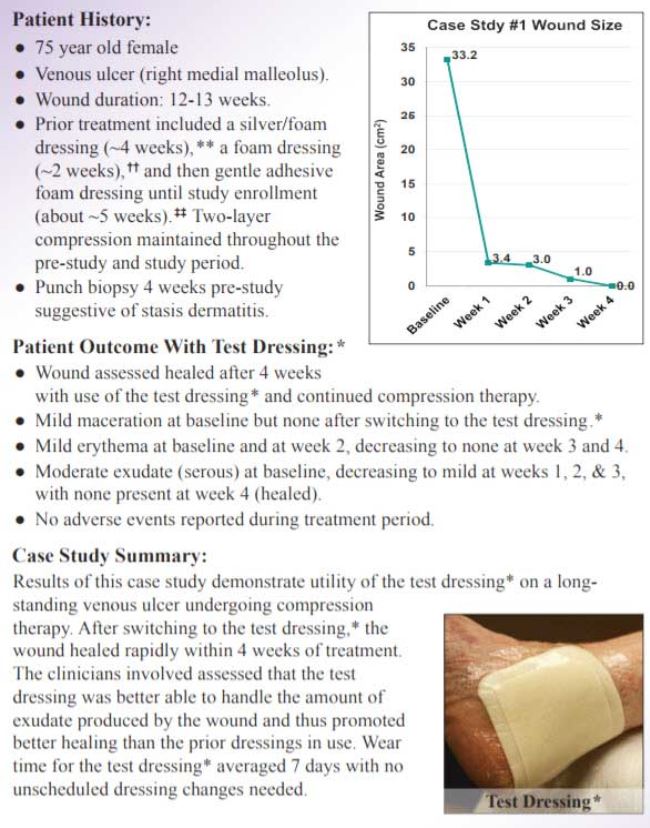
Case Study 1
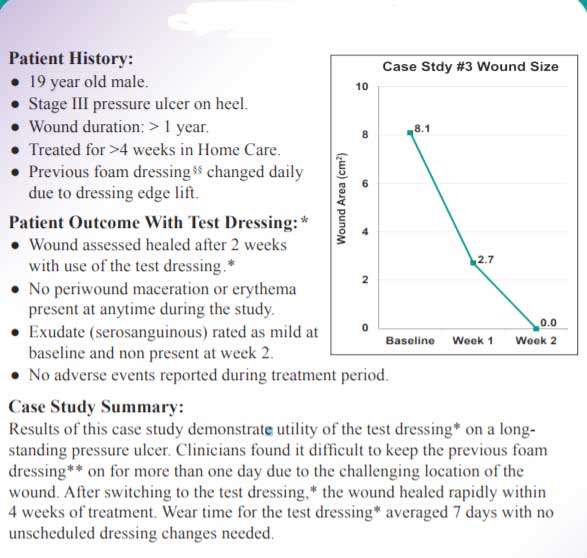
Case Study 2
In Vitro and Healthy Human Studies Assess Foam Adhesive Dressing Breathability and Fluid Handling Properties
Background
Recently, a foam dressing was redesigned to allow it to be more responsive to the degree of moisture present in the wound. This dressing (Dressing A)?* differs from its predecessor and other marketed foam dressings in that it incorporates a new moisture control layer that modulates moisture vapor permeability through the backing under varying wound conditions.
Objective: To assess breathability and fluid handling properties of several marketed foam adhesive wound dressings under simulated high moisture conditions using two in vitro (laboratory) methods and one in vivo (human) test method.
Laboratory Moisture Vapor Transmission Rates (MVTR)
The in vitro MVTR of seven test dressings?* were determined per European Standard EN 13726-2. This in vitro method determined both the "wet" and "dry" MVTR of the dressings. During the wet procedure, the apparatus was inverted and the dressings were in contact with the test fluid, thus determining the MVTR of the dressings when placed over highly exudating wounds. During the dry method, the apparatus was left upright and the dressings were in contact with only the water vapor above the test fluid, thus determining the MVTR of the dressings on dry or low exudating wounds. For all seven test dressings, three lots of each dressing with a minimum of 5 replicates per lot were tested. Additional replicates of Dressings A and C were performed as internal controls within the various tests.
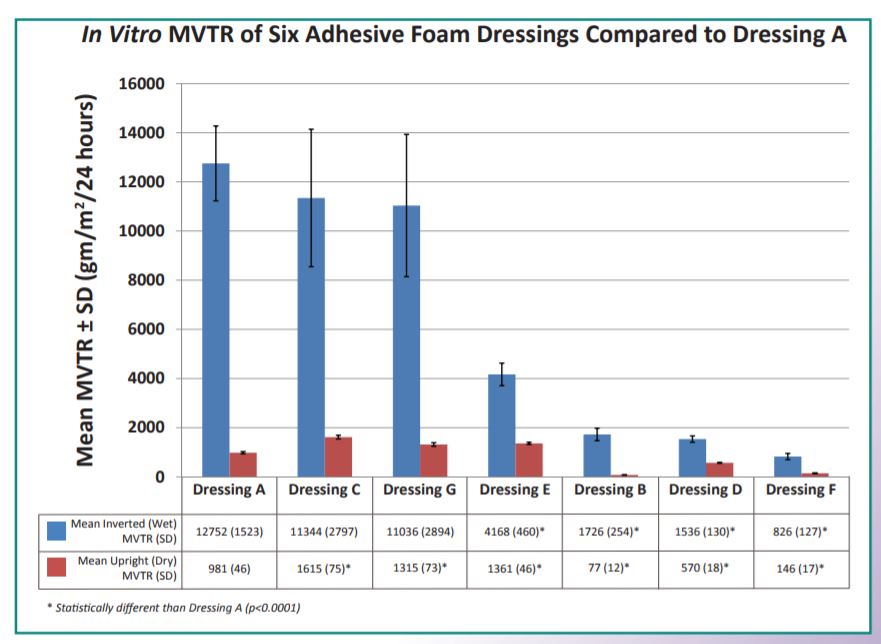
Laboratory Fluid Handling
Fluid handling performance of foam adhesive dressings C?*, D?*, and E?* were compared to Dressing A* with a novel in vitro procedure. Using time lapse photography, side by side comparisons were made hourly in the weights gains of Dressing A and three comparator dressings as the dressings were continuously fed 0.75 ml/hour of an isotonic test fluid (EN 13726-1) over the course of 7 days. The test was conducted at 23 2 degree celcius and 50% 5% relative humidity. For each dressing type, a minimum of three dressings from three different manufacturing lots were tested.
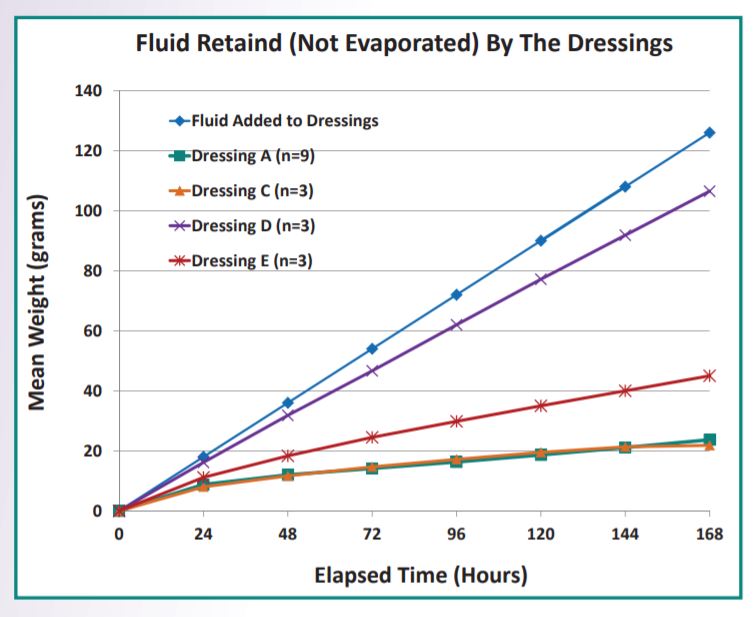
Human Fluid Handling
Artificial Wound Model
Artificial wounds were constructed on the lower backs of healthy human subjects and the test dressings were placed over the artificial wounds. Twelve 1.0 ml injections of artificial wound fluid (AWF) were infused into the artificial wounds at intervals no less than 1 hour apart. This dose of AWF was chosen to model a highly exudating wound. A paired design was utilized with each subject receiving two test dressings alternately placed on the left and right sides of the lumbar region. Six individual studies with 12 to 24 subjects each were performed, with Dressing A serving as a control in each of the studies. The dressings were worn continuously for up to 7 days (plus 3 injections on day 8) or until failure of the dressing. Dressing A absorbed significantly more AWF prior to failure (p7 days, whereas the other test dressings ranged from 1.0 to 3.5 days.
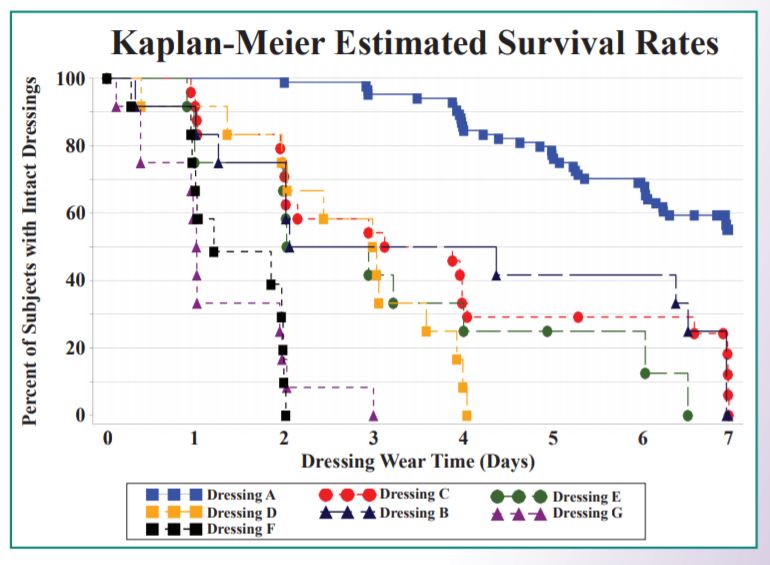
Impact on Cost: Adhesive Foam Wound Dressing Comparisons in Home Care, Long-Term Care, and Hospital Settings
Background
Proper management of exuding wounds is paramount to optimal wound healing. Fluid handling capacity, including absorption and breathability, as well as dressing size and conformability are critical factors in determining the most appropriate wound dressing. Acute and chronic wounds can be challenging and costly to manage. Ideally, foam adhesive dressings should be able to optimize the moist wound environment and reduce the cost burden associated with their use.
Objective: Three models were developed to estimate the economic impact of various foam adhesive dressings being used in home care, long-term care, and hospital settings.
Methods
- An economic model was developed using inputs of:
- Dressing and supply cost from HPIS/Global Healthcare Exchange
- Dressing wear time based on healthy human studies using artificial wound fluid simulating a moderate to high-exudating wound
- Nursing labor cost based on RN Bureau of Labor Statistics
- Dressing change time in minutes based on expert opinion
Costs were calculated for a 60-day treatment period for home care, 30-day treatment period for long term care, and 7-day treatment period for hospital settings.
Figure 1: Artificial Wound Model Procedure:
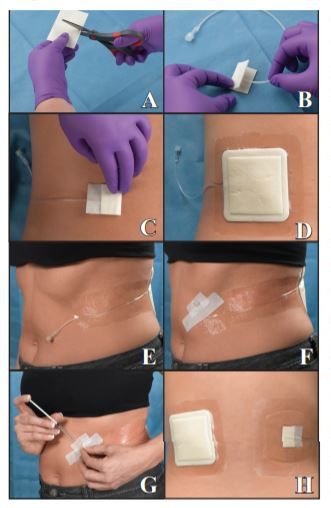
A)A non-stick pad was cut to the desired width but double the length of final wound size.
B)The catheter tip was inserted into the folded pad.
C)The pad was taped into a sandwich around the catheter using a strip of paper tape and gently adhered to the test site.
D)The test dressing was applied over the wound model.
E)Catheter tubing was secured with transparent dressings, providing easy access to the catheter hub.
F)The catheter hub/plug was secured with a strip of cloth tape.
G)Artificial wound fluid (0.1 ml) was injected into the model 12X per day at hourly intervals.
H)Finished wound models showing details of the model through a transparent absorbent dressing. Dressings were assessed until failure for up to 7 days of wear. Failure was defined as dressing fall-off, adhesive border lift sufficient to cause leakage, migration or delamination of dressing layers.

3M Tegaderm High Performance Foam Adhesive Dressing Frequently Asked Questions
Q: How do Tegaderm High Performance Foam Adhesive Dressings reduce the potential for maceration?
A: Inadequate management of wound exudate or moisture can result in periwound maceration. Maceration, a softening of the surrounding tissue, may cause an increased risk of infection and/or prolonged healing. The technology of Tegaderm High Performance Foam Adhesive Dressings is such that the management of moisture is ideal for low- to high-draining wounds. In high moisture wound environments there is rapid vertical wicking of wound exudate into the soft, absorbent foam layer and through to the second layer of the dressing. The second layer of the dressing, the high-capacity absorbent layer, absorbs and retains moisture away from the wound minimizing backward moisture migration. Distribution of exudate across the moisture control layer facilitates evaporation of moisture from the wound through the highly breathable top layer of Tegaderm film.
Q: How does the dressing prevent desiccation in low-draining wounds?
A: In a low-exudating wound, the unique construction of Tegaderm High Performance Foam Adhesive Dressing limits the evaporation of moisture out of the dressing with its moisture control layer thereby helping to maintain a moist wound-healing environment, which is necessary for wound healing.
Q: Is the Moisture Control Layer gauze?
A: No. The Moisture Control Layer may look like gauze; however it is an absorbent, non-woven material integrated with a unique technology to change the breathability of the Tegaderm film layer in the presence of moisture, to balance the moisture in the dressing and provide an optimal wound healing environment.
Q: Can Tegaderm High Performance Foam Adhesive Dressing be left on for seven (7) days?
A: Yes. Tegaderm High Performance Foam Adhesive Dressing may be worn up to 7 days when clinically appropriate. As the dressing absorbs wound drainage, the exudate will wick toward the top layers of the dressing reducing the need for unnecessary dressing changes. Clinical testing results showed that Tegaderm High Performance Foam Adhesive Dressing has significantly higher fluid handling and wear time capabilities then six other adhesive foam dressings tested. Tegaderm High Performance Foam Adhesive Dressing will continue to provide total fluid management even when exudate has reached the outer edge of the foam pad.
Q: When do I need to change Tegaderm High Performance Foam Adhesive Dressing?
A: Indications to change the dressing include:
- a) Visible drainage under the adhesive border.
- b) The edges of the adhesive border are lifting and/or rolling.
- c) The dressing becomes loose, soiled or compromised in any way.
- d) Every seven (7) days or according to facility protocol.
Q: Can Tegaderm High Performance Foam Adhesive Dressings be used with other products?
A: Yes. Tegaderm High Performance Foam Adhesive Dressings may be used as a primary dressing or a secondary dressing with appropriate primary dressings such as 3M Tegaderm Ag Mesh Dressing with Silver, 3M Tegaderm Alginate Ag Silver Dressing, and 3M Tegaderm High Integrity or High Gelling Alginate Dressings. Tegaderm High Performance Foam Adhesive Dressings may also be used with 3M Coban 2 Layer Compression System and 3M Coban 2 Layer Lite Compression System. 3M Cavilon No Sing Barrier Film may be used to protect periwound skin from wound exudate or adhesives prior to dressing application.
Q: Does Tegaderm High Performance Foam Adhesive Dressing minimize friction and shear from clothing, bedding and other surfaces?
A: Yes. Tegaderm High Performance Foam Adhesive Dressings are constructed with a top layer of Tegaderm transparent film providing a low-friction backing which helps to minimize friction and shear forces to vulnerable body areas such as heels, the sacrum and coccyx, and bony areas on the spine.
Q: Do Tegaderm High Performance Foam Adhesive Dressings provide barrier protection against contamination, including bacteria, and viruses?
A. Yes. Tegaderm High Performance Foam Adhesive Dressings are constructed with a top layer of Tegaderm transparent film which when intact and secured to the skin, provides a barrier that allows skin to function normally, but prevents wound exudate strikethrough and acts as a barrier to outside contamination, including bacteria and viruses.*
*In vitro testing shows that the transparent film dressing provides a viral barrier from viruses 27nm in diameter or larger while the dressing remains intact without leakage.
Q: Can Tegaderm High Performance Foam Adhesive Dressings be worn in the shower?
A: Yes: The dressings provide a waterproof seal that allows for showering when the dressings are sealed and intact.
Q: Can Tegaderm High Performance Foam Adhesive Dressings be used over infected wounds?
A: Yes. Tegaderm High Performance Foam Adhesive Dressings may be used on infected wounds under the care of a health care professional. See package insert for additional information.
Q: Can Tegaderm High Performance Foam Adhesive Dressings be used with hyperbaric treatments?
A: While there are no current test standards to qualify materials for use in HBO chambers, 3M has worked with an outside professional testing company to identify suitable tests to evaluate the oxygen compatibility of select 3M skin and wound care products by determining the oxygen index (OI) of the products and the response of the products to oxygen exposure testing. Results of this testing support the conclusion that the 3M products tested did not show any evidence of increased flammability when compared to a suitable control material in high oxygen use environments.
Q: Are there any precautions to consider before using Tegaderm High Performance Foam Adhesive Dressings?
A: Do not use Tegaderm High Performance Foam Adhesive Dressings in combination with oxidizing agents such as hydrogen peroxide or hypochlorite solution. These solutions may affect the structure and performance of the polyurethane foam.
Specifications

3M #90614, Tegaderm Foam Dressing 2.75x3 10/BX, 4 BX/CS
$50.28 per BOX

3M #90612, Dressing Tegaderm Foam Sq 5-5/8x5-5/8" Adhesive 10/Bx, 4 BX/CA
$269.88 per CASE

3M #90614, Tegaderm High Performance Foam Adhesive Dressing, Mini Oval, 2 " x 3", 10/BX, 4 BX/CS
$205.78 per CASE

3M #90611, Dressing Tegaderm Island Latex Fm 4x4-1/2" 2-1/2x3" Pad Adh 10/Bx, 4 BX/CA
$294.68 per CASE


