-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

3M A1841 - STRIP, CLOSURE, SKIN, ANTIMICROBIAL, 1/4"X3", 200 EA/CS, 4 BX/CS

3M Steri-Strip Antimicrobial Skin Closures A1841, 1/4 in. x 3 in./6mm x 75mm, 3 Closures/Envelope, 50 Envelopes/Box, 4 Boxes/Cases
Offers wound closure and support with a special adhesive containing Iodophor.
3M Steri-Strip Antimicrobial Skin Closures are made of porous, nonwoven material. They are reinforced with filaments for strength and are coated with an iodophor-containing adhesive. 3M Steri-Strip Antimicrobial Skin Closures are a small, but effective way to help reduce the risk of surgical site infections on patients. Provides added protection against surgical site infections, with a unique adhesive containing iodophor. Ideal for closure of lacerations, surgical incisions and wound support following early suture or staple removal.
Offers wound closure and support with a special adhesive containing Iodophor. In vitro testing shows the iodophor adhesive used in the manufacture of 3M Steri-Strip Antimicrobial Skin Closures has broad spectrum activity against the most common pathogens causing surgical site infections.
3M offers a full line of 3M Steri-Strip Adhesive Skin Closures that have been clinically proven in numerous studies to help stop surgical site infections at the most ideal time: Before They Start. For more than a century, people worldwide have looked to 3M for new products and ideas that solve everyday problems and make their lives easier and better. For decades, hospitals, clinics, and laboratories have trusted 3M as a dedicated partner supplying essential products designed to meet the unique needs of health professionals, thereby contributing to the advancement of better health practices, outcomes and quality of care.
Using Adhesive Skin Closures Effectively
Which 3M Steri-Strip Skin Closure should I use for wounds that provides reinforced support and tensile strength?
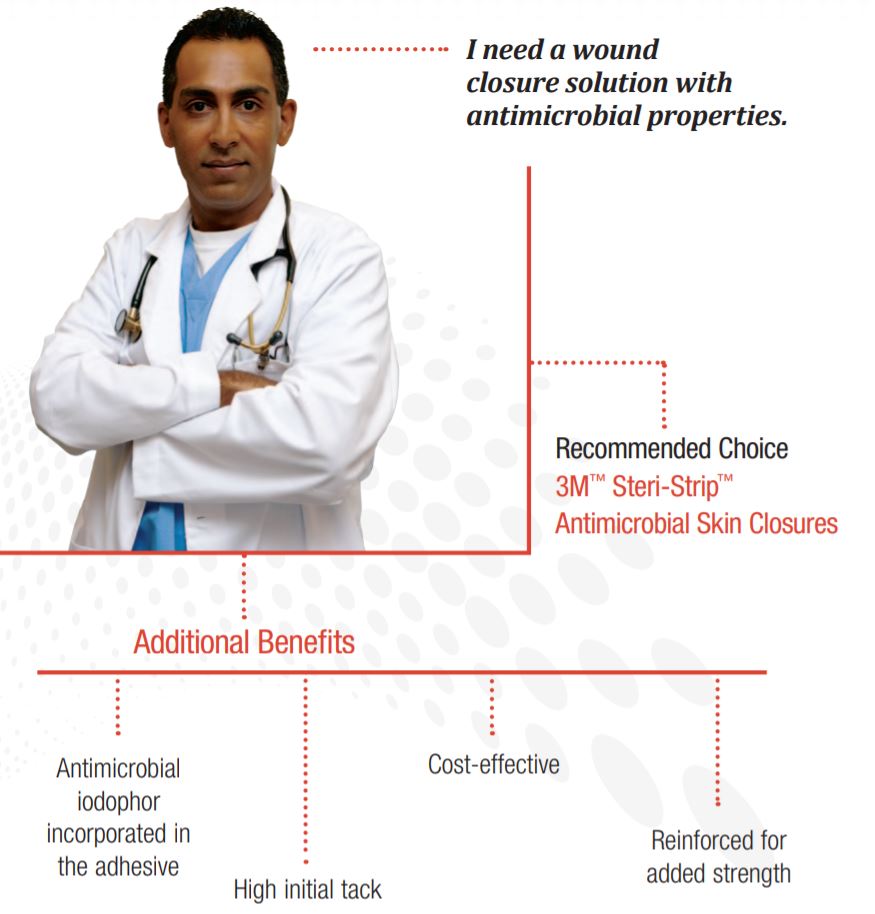
Features and Benefits
Helps reduces infection risk
Iodophor adhesive provides broad spectrum activity against the most common pathogens causing SSIs (Reference on file)
Stays in place
Excellent long-term adhesion, to ensure wounds stay closed while healing.
Easy on patients
Breathable and comfortable to wear. Adhesive is hypoallergenic.
Easy to apply
Fast application
Suggested Applications
Steri-Strip Blend Tone Skin Closures can be used when there is a need for a non-reinforced, gentle closure. It allows natural skin tone to show through, making it ideal for face and hand applications. Same day surgery centers.
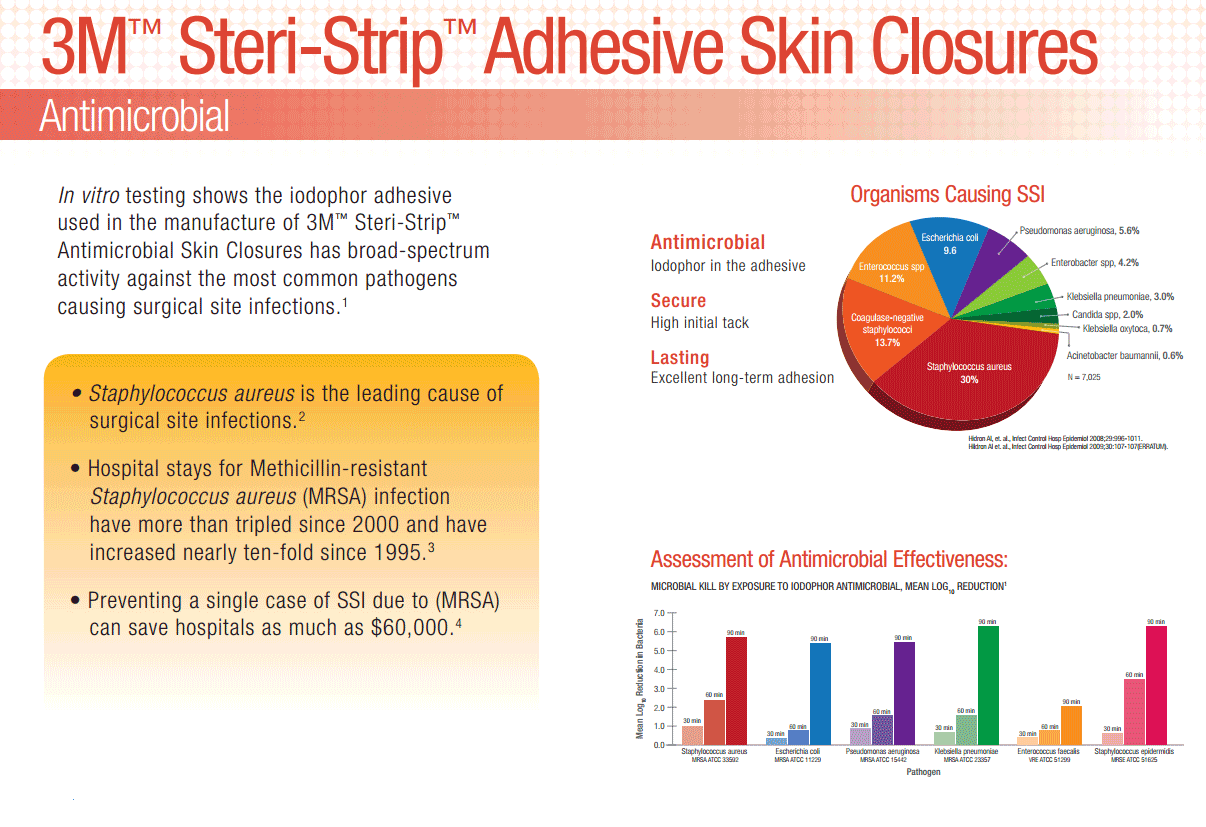
Despite modern advancements in infection prevention, health care-acquired infections (HAIs) remain one of the top 10 leading causes of death in the United States and are responsible for nearly 100,000 deaths each year.1 Surgical site infections (SSIs) are the second most-common HAI, accounting for 20% of all HAIs among hospitalized patients. Post-operative SSIs are the most common healthcare-associated infection in surgical patients occurring in up to 5 percent of surgical patients.

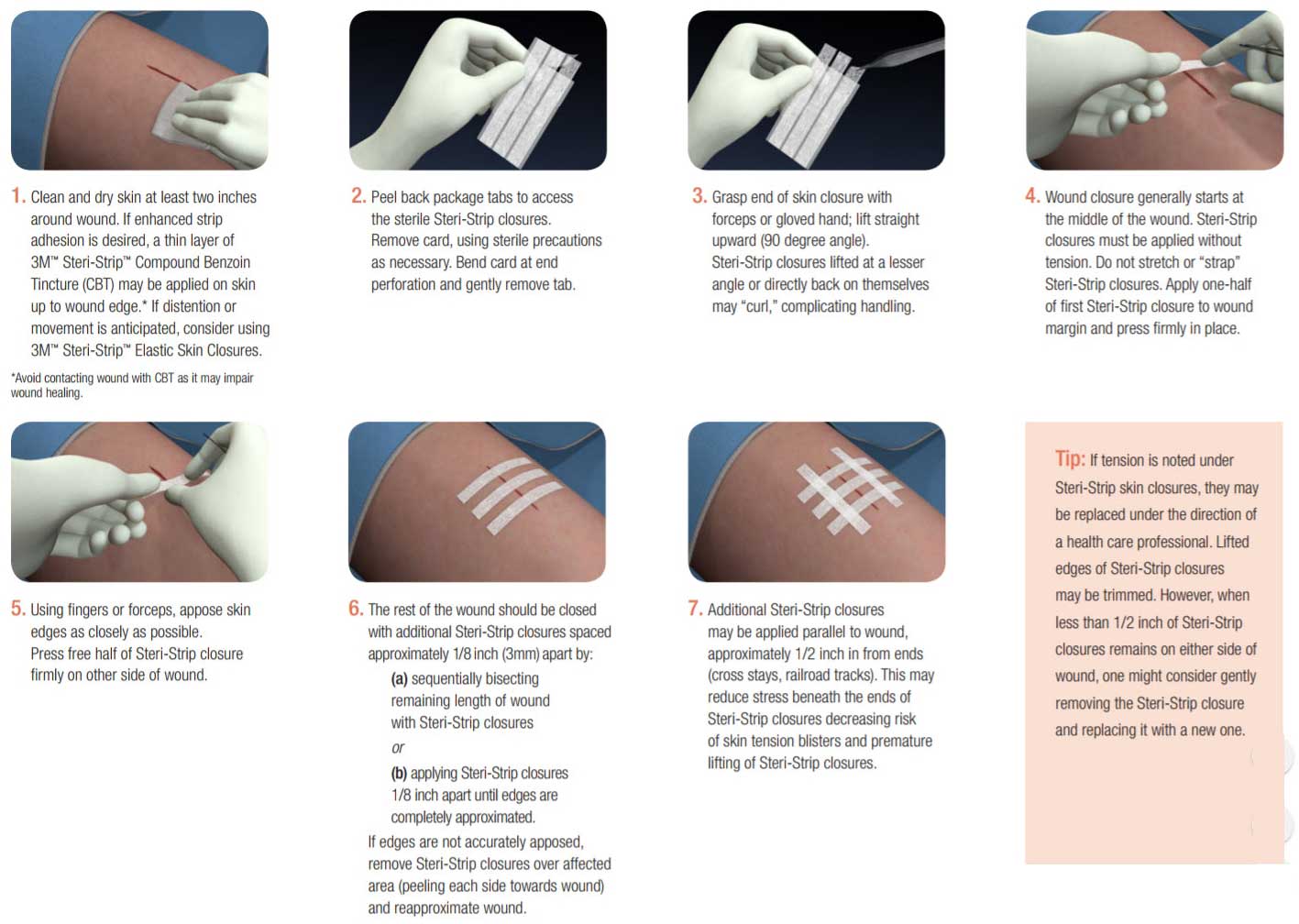
Skin Closure Application
Skin Closure Removal

3M Steri-Strip Blend Tone Closures(non-reinforced)| Commonly Asked Questions
1. Components
Question: Do Steri-Strip Antimicrobial skin closures contain latex?
Answer: No. Neither the packaging nor the Steri-Strip Antimicrobial skin closures contain natural rubber latex.
Question: Do Steri-Strip Antimicrobial skin closures contain fiberglass?
Answer: No. Steri-Strips skin closures have never contained fiberglass. The reinforcing filaments in the reinforced Steri-Strip skin closures and Steri-Strip Antimicrobial skin closures are made of polyester.
Question: Are Steri-Strip Antimicrobial skin closures hypoallergenic?
Answer: No. The Steri-Strip Skin Antimicrobial skin closures contain an iodophor compound, so they are not recommended for use on patients with known sensitivity to iodine.
Question: What is the shelf life of Steri-Strip Antimicrobial skin closures?
Answer: 5 years. The expiration date may be found on the top of each Steri-Strip skin closure package. Without breaking the seal, fold the rear package flap back. Holding the package up to the light, you will note: LOT, hourglass, year, 1-2 numbers, several letters The hourglass symbol represents the expiration date. 2004-10 would indicate that the product will expire in October of 2004.
Question: Are Steri-Strip Antimicrobial skin closures sterile? Can Steri-Strip Antimicrobial skin closures be resterilized?
Answer: Steri-Strip Antimicrobial skin closures are sold sterile. They may NOT be resterilized with ethylene oxide.
2. Selection and application
Question: What is the best way to get great adhesion of Steri-Strip skin closures?
Answer: Steri-Strip skin closures adhere best to clean, dry skin. This can be accomplished by ensuring that bleeding is under control and cleaning the skin with saline or alcohol to remove oils or exudates followed by gentle drying of the skin surface. Steri-Strip closures have a pressure sensitive acrylate adhesive. Adhesion of skin closures will be enhanced if you gently but firmly press or stroke the skin closure strip during application so that the adhesive gets into the nooks and crannies of the skin surface. See application instructions. Steri-Strip skin closures are designed for closure of low-tension wounds. They are contraindicated for use in high-tension wounds that cannot easily be approximated with fingers or forceps or on infected wounds.
Question: Should I use a skin tackifier under Steri-Strip skin closures?
Answer: Compound benzoin tincture (CBT) may be used to increase adhesion of SteriStrip skin closures. Although, CBT does not contain iodine, it may be an irritant to some individuals and should not enter the wound.
Question: What can I do to protect very fragile skin?
Answer: Apply the skin closure to dry, clean skin that is free of chemicals. If hair is to be removed, a clipper should be used. To further protect from adhesive trauma, a skin barrier such as 3M Cavilon No Sting Barrier Film may be applied and allowed to dry thoroughly before applying the skin closure. Avoid chemicals designed to increase adhesion. To reduce the risk of skin stripping, gently remove Steri-Strip skin closures: "low and slow", towards the center of the wound, while supporting the skin.
Question: What could cause blistering of the skin under the ends of skin closure strips?
Answer: The most common cause of blistering is tension. Edema, hematoma, or distention due to bloating may distort the skin surface. If the adhesive is firmly attached to the epidermis and the adhesive closure backing does not "give", the epidermis may be pulled away from the dermis resulting in blisters or skin tears. Blistering is usually noted on both ends of the strip. A similar situation occurs when a joint or other area of movement is covered with a strip that doesnt stretch. If tension is noted, loosen, reposition, or replace the skin closure strips. To decrease the risk of tension injuries:
- Do not use Steri-Strip skin closures on high-tension wounds.
- Apply Steri-Strip skin closures without tension. Do not strap the wound closed.
- Consider 3M Steri-Strip Elastic Skin Closures if edema, hematoma, distention, or movement are anticipated.
- Monitor the wound for evidence of swelling or blistering.
- If tension is noted under Steri-Strip closures, they may be replaced under the direction of a health care professional.
Question: I suspect that the area around the wound may swell. Will this affect the type of skin closure I should use?
Answer: Yes, if expansion or movement (such as over a joint) is anticipated, consider using Steri-Strip Elastic skin closures.
Question: Are reinforced Steri-Strips skin closures safe to use on PICC lines?
Answer: Yes.
3. Length of wear
Question: How long can Steri-Strip skin closures be worn?
Answer: Steri-Strip skin closures are usually worn until they fall off or the healthcare provider removes them. This usually occurs within 5 to 7 days for the standard reinforced Steri-Strip skin closures. Wear time may vary depending on area of the body, skin type, degree of friction to that area, etc. Some health care providers prefer to remove, and sometimes replace, the SteriStrip skin closures, on follow-up visits. The timing of strip removal and the length of continued reinforcement and support of the wound with skin closure strips is left to the discretion of the health care provider.
Question: If skin closure ends loosen, should they be trimmed?
Answer: Yes. Once the edges loosen, a skin closure strip is more likely come off than if the strip is intact. If the loosened ends of the strips are trimmed so that they do not catch on clothing, the duration of adherence is extended. However, when less than 1/2 inch of the strip remains on either side of the wound, health care providers may want to consider gently removing the strip and replacing it with a new strip.
Question: Can I get Steri-Strip skin closures wet?
Answer: Unless contraindicated, you may shower 24 hours after the Steri-Strip skin closures have been applied. Then pat the strips dry. However, some studies indicate that strip adherence may be improved when bathing is limited. For additional protection, you may cover the Steri-Strip skin closure with a 3M Tegaderm Transparent Dressing.
Specifications


Medline #NON250314, STRIP, CLOSURE, WOUND, MEDI-STRIP, 1/4"X3", 600 EA/CS, 200 PK/CS, 4 BX/CS
Call for Pricing

3M #R1541, STRIP, CLOSURE, SKIN, REINFORCED, 1/4"X3", 200 EA/CS, 4 BX/CS
$84.00 per CASE

3M #A1847, STRIP, CLOSURE, SKIN, ANTIMICROBIAL, 1/2"X4", 200 EA/CS, 4 BX/CS
$393.17 per CASE

3M #E4541, STRIP, CLOSURE, SKIN, ELASTIC, 1/4"X3", 200 PK/CS, 4 BX/CS
$246.01 per CASE

