-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

BD 515003 - INJECTOR N35 PHASEAL, 200 EA/CS

BD PhaSeal Syringe Safety Device
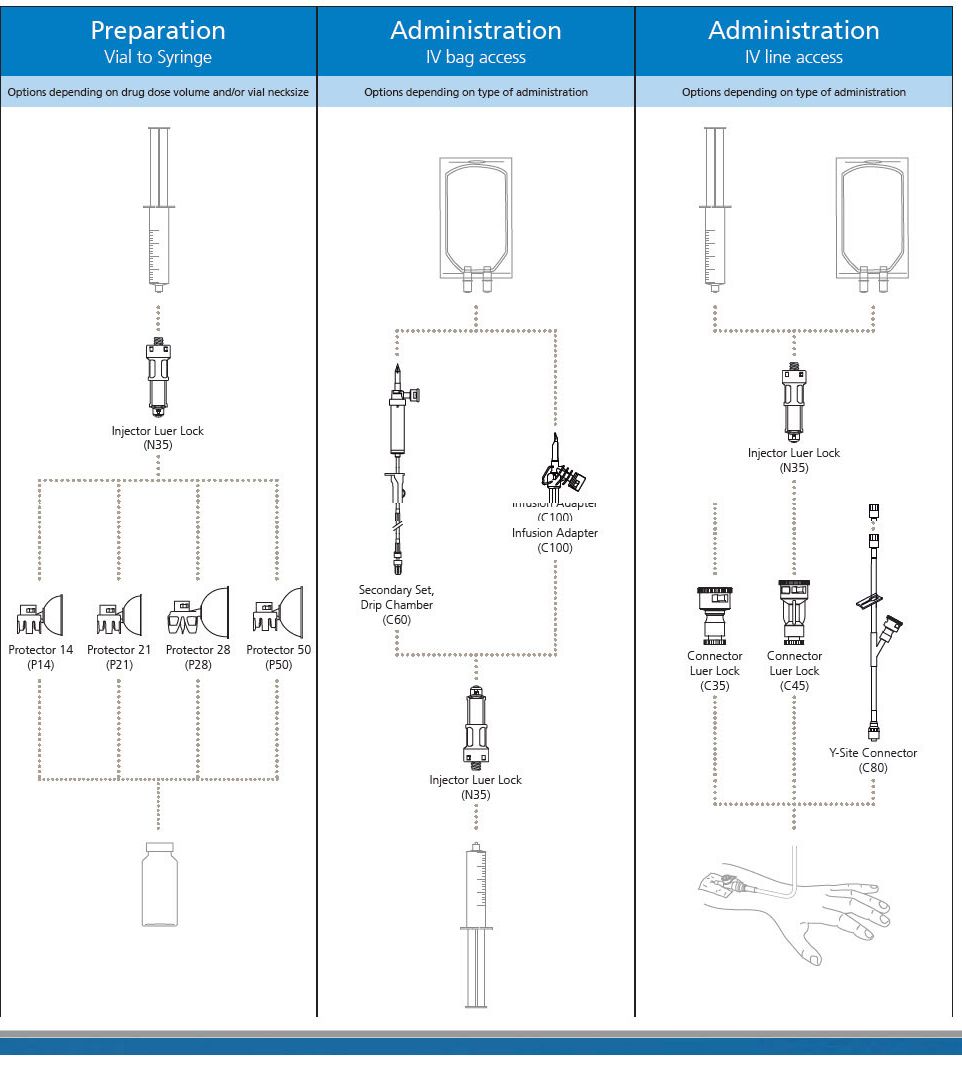
BD PhaSeal is a clinically proven closed-system drug transfer device that prevents hazardous drug interaction exposure. Whether you are to prepare a drug for administration by infusion or by injection, the BD PhaSeal system offers the range of products to meet your safe handling requirements.
BD PhaSeal Injector
The BD PhaSeal Injector attaches a syringe to the BD PhaSeal drug vial access device on a drug vial, or a syringe to BD PhaSeal IV line access devices on IV tubing forming dry, leak-proof connections during drug preparation and administration.
The Injector ensures a closed transfer of the drug by means of double, elastomeric membranes.

BD PhaSeal System
The BD PhaSealsystem is a closed system drug transfer device (CSTD) that has been demonstrated to prevent exposure to hazardous drugs, from drug preparation to IV administration.
It is an airtight, leakproof system that utilizes a membrane-to-membrane technology. It mechanically prohibits the transfer of environmental contaminants into the system and the escape of drug or vapor concentrations outside the system, thereby minimizing individual and environmental exposure to drug vapor aerosols and spills. It also prevents microbial ingress within an ISO Class V environment with proper aseptic technique. The BD PhaSeal system is a pioneer in the CSTD space. With more than 25 peer-reviewed, published studies, it is the most studied and according to a recent U.S. survey, BD offers the most widely used CSTD portfolio in the US.
Key Product Features
| CE Mark | Product is CE-marked |
| External fitting | Luer lock |
| Dimensions | 71 mm x 17 mm |
| Cannula volume | 0.04 mL |
| Material | Not reusable; BPA free; latex free; sterilized using ethylene oxide |
| Cannula | Stainless steel |
| Cylinder | Acrylonitrile-butadiene-styrene copolymer |
| Membrane | Thermoplastic elastomer |
| Piston/Cannula housing | Polypropylene |
| Safety sleeve | Polyoxymethylene |
| Piston sealing | Silicone |
| Total Shelf Life | 913 |
| Sterile | Sterilized product |
| Safety Engineered | Safety engineered product |
| Safety Engineered Feature | Passive needle shielding device, Closed system drug transfer |
| Sterilization Method | EO |
| BPA Free | Not made with BPA |
| DEHP Free | Not made with DEHP |
| Latex Statement | Not made with natural rubber latex |
| PVC Free | Not made with PVC |
| Disposable | Disposable product |
| Single Use | Product is for single use only |
Product Packaging Information
| Packaging Level | Shelfpack | Case | Each |
| Quantity | 50 | 200 | 1 |
| Length | 277.0 mm | 561.34 mm | |
| Width | 185.0 mm | 383.54 mm | |
| Height | 117.0 mm | 132.08 mm | |
| Weight | 629.25 g | 2.517 kg | 12.585 g |

BD #515003, INJECTOR N35 PHASEAL, 200 EA/CS
$1,731.06 per CASE

BD #515003, Injector Luer Lock N35 50/Box 4 Box/Ca
$2,265.46 per CASE

BD #515003, BD PHASEAL INJECTOR LUER LOCK (N35) 50/BX
Call for Pricing

BD #515003, Injector Luer Lock N35 200/Ca
$1,043.63 per CASE