-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Smith & Nephew 66800075 - Allevyn Ag Adhesive 10 cm x 10 cm. Revised.

ALLEVYN Ag Adhesive - Advanced Foam Wound Dressings, 10.cm x 10cm
A highly absorbent secure antimicrobial foam dressing that is also available in a sacral shape for this area that is at high risk of contamination.
ALLEVYN Ag Adhesive is an antimicrobial absorbent 3-layer dressing consisting of the following:
- A SSD (Silver Sulfadiazine) containing absorbent hydrocellular pad sandwiched between a perforated adhesive wound contact layer and a waterproof out film.

The dressing provides an effective barrier to microbial contamination protecting the wound from invasive pathogenic microorganisms, thus assisting faster healing. SSD is effective against microorganisms present in the dressing and in the wound. In the presence of exudate the dressing will help maintain a moist wound environment.
- The unique triple action technology helps to maintain an optimal balance in fluid creating a moist wound environment for healing
- The low allergy adhesive helps to keep the dressing securely in place without adhering to the wound
- The highly breathable top film helps to minimise the risk of maceration to the wound and peri-wound
- Provides an effective barrier function to exogenous bacteria as well as helping to prevent fluid and bacterial strikethrough
- Easy to apply and remove
- Conforms to body contours and is very comfortable for patients

Technology
ALLEVYN Adhesive consists of a highly absorbent hydrocellular foam pad held between an adhesive perforated wound contact layer and a highly permeable outer top film.
Breathable Top Film
The outer polyurethane top film switches to become more breathable in the presence of exudate. It also helps to prevent strikethrough and provides an effective barrier to bacteria and minimises the risk of cross contamination.
Highly Absorbent Foam Core
The hydrocellular foam core is highly absorbent, providing absorption capacity for up to 7 days.
Non-Adherent Wound Contact Layer
The wound contact layer has a low allergy based adhesive, which adheres well to intact skin, will not stick to the wound and has a proven low risk of skin irritation.
The unique triple layer technology effectively manages exudate helping to ensure that the wound is kept at optimum moisture levels to support moist wound healing. It does this by:
- Transpiring enough fluid to keep the dressing comfortable and conformable.
- Retaining enough fluid to keep a healthily moist wound environment.
- Absorbing enough fluid to remove unwanted exudate and cellular debris.
ALLEVYN Ag Adhesive is a dressing that delivers fluid management via the unique triple-action technology which absorbs, retains and transpires the optimal balance of fluid. This optimises the moist wound environment and helps to promote healing . The addition of silver sulfadiazine to the hydrocellular pad provides a sustained antimicrobial action (demonstrated in-vitro) .

- A highly breathable top film helps to minimise the risk of maceration to the wound and peri-wound as well as providing an effective barrier to bacteria to minimise the risk of contamination
- Low allergy adhesive helps to keep the dressing securely in place without adhering to the wound
- High conformability to body contours helping to ensure patient comfort
- Proven in-vitro against a broad spectrum of bacteria including antibiotic resistant bacteria such as Pseudomonas, Methicillin-Resistant Staphylococcus Aureus (MRSA) and Vancomycin Resistant Enterococcus (VRE)
- .Provides a sustained action with antimicrobial properties remaining effective for up to 7 days in-vitro
Indications
ALLEVYN Ag Adhesive is an antimicrobial absorbent dressing for the management, by secondary intention of chronic and acute full thickness, partial thickness or shallow granulating wounds, exudating wounds such as:
- pressure ulcers
- venous ulcers
- diabetic ulcers
- burns
- donor sites
- fungating / malignant wounds
- surgical dehisced wounds
ALLEVYN Ag Adhesive may be used on infected wounds. Where the product is used on infected wounds the wounds should be treated as per local protocol.
Application
- Cleanse the wound in accordance with normal procedures.
- Select appropriate dressing size. ALLEVYN Ag Adhesive can be cut to fit awkward areas. Always use a clean technique when cutting the dressing.
- Prepare and clean the skin surrounding the wound area and remove excess moisture. Any excess hair should be clipped to ensure close approximation to the wound.
- Remove one of the protector films and anchor the adhesive side of the dressing to the skin.
- Remove the remaining protector film and smooth the dressing over the remainder of the wound without stretching, ensuring there are no creases. The pad area of the dressing must be adhered across the entire surface of the wound.
- If the dressing has been cut ensure any exposed foam areas are covered with an appropriate film dressing taking care not to cover the entire dressing.
- When positioning ALLEVYN Ag Adhesive Sacrum place the narrow end of the dressing a minimum of 2 cm above the anal sphincter, then smooth the dressing over the sacrum
Frequency of Change
During the early stages of wound management ALLEVYN Ag Adhesive should be inspected according to local clinical protocol. Where the product is used on infected wounds the infection should be inspected and treated as per local clinical protocol. Dressings can be left in place for up to 7 days, except the sacral area where dressings can be left in place for up to 5 days, depending on the condition of the wound and the surrounding skin or until exudate is visible and approaches to within 0.5cm of the edge of the dressing pad, whichever is sooner. The decision of when to change should be dependent upon clinical assessment and local protocols should also be taken into consideration.
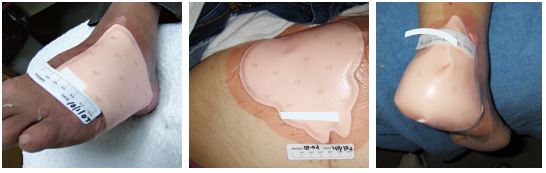
Contra-indications
- Do not use on patients known to be hypersensitive to silver sulfadiazine or sulfonamides
- As sulfonamides are known to cause kernicterus, ALLEVYN Ag Adhesive should not be used on females who are at, or near term pregnancy or lactating, on premature infants or on newborn infants during the first month of life.
Precautions
- For external use only
- ALLEVYN Ag Adhesive is not compatible with oxidizing agents (e.g. EUSOL) or hydrogen peroxide, as these can break down the absorbent polyurethane component of the dressing.
- ALLEVYN Ag Adhesive may not be compatible with topical antimicrobials.
- In the event of clinical infection, topical silver does not replace the need for systemic therapy or other adequate infection treatment.
- In common with all adhesive products, some cases of irritation and/or maceration of the skin surrounding the wound may be observed.
- It should be noted that inappropriate use or too frequent dressing changes, particularly on patients with fragile skin may result in skin stripping.
- Avoid contact with electrodes or conductive gels during electronic measurements e.g. EEG and ECG.
- When ALLEVYN Ag Adhesive is used on a patient during MRI (Magnetic Resonance Imaging) examination some warming may be experienced.
- ALLEVYN Ag Adhesive is not compatible with oil based products such as petrolatum.
- ALLEVYN Ag Adhesive is not suitable for use alone on cavity wounds but may be used over cavity wounds as a secondary dressing.
- As with all products containing silver sulfadiazine the following precautions should be noted, especially when covering a large surface area with the dressing,
- Use caution in patients with significant hepatic or renal impairment,
- Use caution in individuals known to have glucose-6-phosphate dehydrogenase deficiency,
- Effects of systemically administered drugs may be altered. This can especially apply to oral hypoglycaemic agents and to phenytoin. In the case of these drugs, it is recommended that blood levels should be monitored as their effect can be potentiated.

Smith & Nephew #66800086, Allevyn Ag non Adhesive 10 cm x 10 cm.
$90.47 per BOX

Smith & Nephew #66157637, Allevyn Non Adhesive 10 cm x 10 cm
$27.53 per BOX

Smith & Nephew #66000599, Allevyn Adhesive 10 x 10 cm, 10 Per/Bx
$33.24 per BOX

Smith & Nephew #66157335, Allevyn Non Adhesive 10 cm x 20 cm (10/BX)
$47.73 per BOX

