-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Smith & Nephew 66800098 - ALLEVYN AG HEEL

ALLEVYN Ag Heel - Absorbent Silver Barrier Dressing, 10.5cm x 13.5cm Heel
ALLEVYN Ag Heel Absorbent Silver Barrier Dressing
- Optimal exudate management
- Easy to use range of dressing sizes and can be left in place for up to 7 days depending on the nature of the wound and the level of exudate present
- Breathable top film
- Foam core
- Non-adherent wound contact layer
- Profiled edges
Benefits
- Reduces risk of excess fluid at the wound site, excess wound fluid is transpired away more quickly.
- Promotes rapid wound closure, reduced risk of maceration, less potential for leakage and odor, longer wear-time.
- Reduces bacterial load (in-vitro).
- Removes some barriers to healing allowing the host to regain control and progress the wound to closure.
- Product is easy to apply, can be removed in one piece, and is designed in sizes & shapes appropriate for target wound types.
- Adhesive products offer an all-in-one solution -no need for secondary retention.
- Provides a convenient, efficient, cost effective solution for wound management.
- Top film switches to become more breathable in the presence of fluid, therefore it responds, when the clinician needs it most.
- Provides an effective barrier to bacteria penetration
- Waterproof
- Conformable and comfortable.
- Provides absorption capacity for up to 7 days.
- Prevents healing tissue from growing into the dressing minimizing pain on removal.
- Maintains a moist wound interface.
- Low allergy adhesive.
- Protects from leakage
Technology
ALLEVYN Non-Adhesive utilises the unique triple action technology seen across the ALLEVYN range of dressings. By combining an absorbent hydrocellular pad with a non-adherent wound contact layer and breathable top film; the dressing manages fluid to maintain optimal moist wound healing conditions.
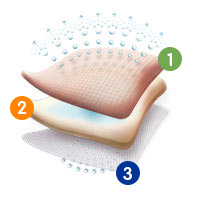
Breathable Top Film
The outer polyurethane top film is highly breathable, helps to prevent strikethrough and provides an effective barrier to bacteria.
Highly Absorbent Foam Core
The hydrocellular foam core is highly absorbent
Non-Adherent Wound Contact Layer
The wound contact layer will not stick to the wound and is suitable for fragile skin.
ALLEVYN Non-Adhesive is ideal for fragile and sensitive skin due to its non-adherent wound contact layer and soft and conformable construction. The specially designed Heel dressing fits closely to the heel area allowing even this most challenging of body parts to be dressed comfortably.
Indications
ALLEVYN Ag Heel Absorbent Silver Barrier Dressing is indicated for exudate absorption and the management of partial to full thickness wounds. Some typical wounds are:
- Ulcers (venous, arterial, diabetic)
- Pressure Sores
- Donor Sites
- Surgical Incisions
- Surgical Excisions
- Burns (1st and 2nd degree)
Creation and maintenance of a moist wound environment. Moist wound environments have been established as optimal environments for the management of the wound. Provides physical separation between the wound and external environments to assist in preventing bacterial contamination of the wound.
Precautions
- For external use only
- ALLEVYN Ag Heel is not compatible with oxidizing agents (e.g. EUSOL) or hydrogen peroxide, as these can break down the absorbent polyurethane component of the dressing
- ALLEVYN Ag Heel dressings are not intended to provide treatment for infected wounds.
- ALLEVYN Ag Heel may be used on infected wounds being managed in accordance with institutional clinical protocols for infection abatement as an adjunct to the standard treatment regimen to provide a barrier to bacterial penetration.
- ALLEVYN Ag Heel may not be compatible with topical anti-microbials
- Reddening of the skin around the wound may be observed. This may be related to irritation of fragile skin or wound exudates remaining in contact with normal skin for prolonged periods of time. If reddening or sensitization occur, discontinue use.
- Avoid contact with electrodes or conductive gels during electronic measurements e.g. EEG and ECG.
- When ALLEVYN Ag Non Adhesive is used on a patient during MRI (Magnetic Resonance Imaging) examination some warming may be experienced
- ALLEVYN Ag Heel is not compatible with oil based products such as petrolatum.
- ALLEVYN Ag Heel is not suitable for use on cavity wounds but may be used over cavity wounds as a secondary dressing.
- As with all products containing silver sulfadiazine the following precautions should be noted, especially when covering a large surface area with the dressing,
- 1. Use caution in patients with significant hepatic or renal impairment,
- 2. Use caution in individuals known to have glucose-6-phosphate dehydrogenase deficiency,
- 3. Effects of systemically administered drugs may be altered. This can especially apply to oral hypoglycaemic agents and to phenytoin. In the case of these drugs, it is recommended that blood levels should be monitored as their effect can be potentiated.

Smith & Nephew #66800456, DRESSING, ALLEVYN AG GENTLE, 4X4, 70/CS
$1,375.33 per CASE

Smith & Nephew #66007630, DRESSING, FOAM, ALLEVYN, HEEL, 4.5"X5.5", EACH
$16.89 per EACH

Smith & Nephew #66020978, DRESSING, AG, NON-ADHESIVE, ALLEVYN, 4"X4", 70 EA/CS
$1,430.34 per CASE

Smith & Nephew #66800458, DRESSING, ALLEVYN AG GENTLE, 6X6, 30 EA/CS
$1,352.20 per CASE

