-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Avanos Medical 01-EC-350-0001 - Restore 2, 7.0-8.5 MM Endotracheal Tube (ETT) Cleaner, 10 EA/CS
Avanos 01-EC-350-0001 Restore 2 Endotracheal Tube (ETT) Cleaner
The AVANOS Restore2 endotracheal tube (ETT) cleaner is a sterile, single-use device. The Restore2 has a radially expanding, squared-edge wiper balloon that can be used to clear away mucus, secretions and bacteria from the inside of the ETT.
Avanos 01-EC-350-0001 Restore 2 Endotracheal Tube (ETT) Cleaner Features
- Sterile, single-use device
- Compatible with all current closed suction systems and all endotracheal tubes sizes 7.0-8.5
- After connection, wiping procedure can be performed while the patient is being ventilated
- Length-limited catheter avoids insertion more than 1-2 cm beyond the ETT tip on standard length tubes
- Atraumatic catheter tip helps prevent injury if it ever contacts tracheal mucosa
- Protective sheath helps prevent exposure to secretions
An adjunct to your closed suction system
The Challenge:
Due to accumulated secretions, performance of an ETT can be equivalent to that of a new ET tube one to four sizes smaller. This may impact the tolerance of ventilator weaning.
The Win:
Restore2. Single use. Multiple Benefits.
- Unique wiping technology clears the ET tube of tenacious secretions and biofilm
- Reduces airway resistance and work of breathing
- Gives patient an improved opportunity to pass their SBT
- Decreased costs associated with care of intubated patients, providing a return on investment
Daily clearing of the ET tube results in a decrease in average vent days.
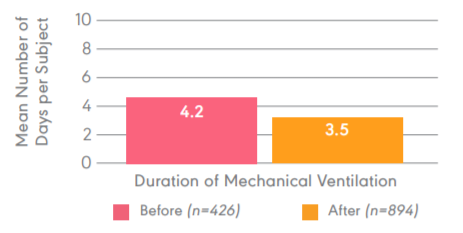
Study objective:Evaluate the efficacy of cleaning the ETT daily with the Restore2 prior to the spontaneous breathing trial. Design: 1,320 patients in a five-year, retrospective, observational, single centered study Primary endpoints: Average duration of mechanical ventilation, average hospital length of stay, and average hospital direct cost per subject.
Results: Decreased average time on the ventilator from 4.2 to 3.5 days (0.7 (+-)0.8, p<0.01).
Unique wiping techonology clears the ET tube to help liberate your patients from the vent sooner

- Compatible with all current closed suction systems and all endotracheal tubes sizes 7.0-8.5
- After connection, wiping procedure can be performed while the patient is being ventilated
- Length-limited catheter avoids insertion more than 1-2 cm beyond the ETT tip on standard length tubes
- Soft, silicone catheter with atraumatic tip
- Protective sheath helps prevent exposure to secretions
- Depth markers on the catheter guide appropriate insertion
Avanos Restore 2 Endotracheal Tube (ETT) Cleaner 01-EC-350-0001 Specifications
| Product Configuration | Disposable |
| Sterile | Yes |
| Sterilization Method | Ethylene Oxide |
| Depth Marks in Centimeters | Yes |
| Catheter Length (cm) | 43.5 |
| Catheter Material | Silicone |
Avanos Liberator, ET Tube Clearing System with Manifold 01-EC-254-1201 Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as ""Not made with natural rubber latex"": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |

