-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Avanos Medical 40-5432 - CORFLO Nasogastric/Nasointestinal Feeding Tube with ENFit Connector, 12FR, 43 in Catherter Length, 91 cm Tube Length, 10 EA/CS
Avanos 40-5432 CORFLO Nasogastric/Nasointestinal Feeding Tube with ENFit Connector
ORFLO Nasogastric/Nasointestinal Feeding Tube with ENFit Connector is uniquely designed for delivering enteral nutrition. Each package comes with a variety of features that provide consistent performance and convenience, including an anti-clog bolus, centimeter markings, and a water activated lubricated tip and internal lumen.Multiple Choices for Clinician ConvenienceWith multiple packages from which to choose, clinicians can choose whether or not they want ENFit, Universal, and Anti-IV proximal connectors. Similarly, our polyurethane CORFLO NG/NI tubes are available in various lengths and French sizes. Avanos Medical, a leader in the enteral feeding market, offers a wide variety of innovative, high-quality enteral feeding tubes and accessories designed for delivering enteral nutrition.
Avanos 40-5432 CORFLO Nasogastric/Nasointestinal Feeding Tube with ENFit Connector Features & Benefits
- Weighted/Non-Weighted
- With and without stylet
- ENFit, Universal, and Anti-IV proximal connectors
- Anti-clog bolus
- CM Markings
- MRI conditional once stylet is removed
- Flow-Through Stylet
- Radiopaque
- Water Activated Lubricated Tip & Internal Lumen (stylet tubes only)
About CORFLO Nasogastric/Nasointestinal Feeding Tube with ENFit Connector
The AVANOS CORFLO Nasogastric/Nasointestinal Feeding Tube is a medical grade polyurethane feeding tube that has been specifically designed for patient comfort and safety during tube insertion and use. It is intended for use in patients who require intermittent or continuous tube feedings via the nasogastric or nasointestinal pathway.
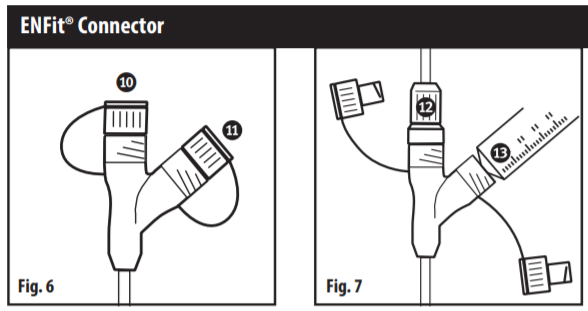
Tube Capped (Fig. 6)
- Feed Port
- Auxiliary Port
Tube Connected (Fig.7)
- Administration Set
- Syringe
Features
- Distinctive YELLOW tube design
- Exit ports:
- Anti-clog bolus
- Bolus
- ENFit design employed for reducing the risk of tubing misconnections.
- ENFit satisfies the ISO Standard (80369-3) for small bore enteral devices.
- Water-activated C-19* lubricant on tube tip
- Dual port to maintain closed system
- For YELLOW feeding tubes Radiopacity: 20% barium tube, 40% barium tip
Ability to Connect to Non-Enteral Medical Devices
The ENFit connectors were designed to prevent misconnections between enteral devices and other devices used in various medical applications. However, the design of the ENFit connector cannot overcome all chances of misconnection. The following connector types are potential misconnections for the ENFit connector (feeding/ medication access port) of this enteral feeding tube:
- Suction ports on Endotracheal Suction Systems
- Respiratory circuit filtration connectors
- Oxygen inlet connectors for Resuscitation Devices
- Baxter IV Solution Bag ports (such as NaCl, Ringers Solution, etc.)
- Sample ports on drainage bags
- Peritoneal Dialysis connectors
- Cones & sockets of ISO 5356-1:2004 & ISO 5356-2:2004
- Temperature sensor connectors & mating ports of ISO 8185:2007
- Oxygen nipples as defined in EN 13544-2:2002
CORFLO NG/NI feeding tubes have satisfied biocompatibility testing as a device for long-term use per ISO 10993-1.
Note: Placement and use of any feeding tube may result in patient discomfort.
Avanos 40-5432 - Indications for Use
The AVANOS CORFLO Feeding Tube is intended for use in those patients who require intermittent or continuous tube feedings via the nasogastric or nasoenteric route.
MRI Safety Information
Non-clinical testing demonstrated that the weighted CORFLO Enteral Feeding Tubes are MR Conditional. A patient with this device can be scanned safely immediately after placement under the following conditions:
Static magnetic field of 3-Tesla or less
Spatial gradient magnetic field of 720-Gauss/cm or less
MR system reported whole body averaged SAR of 3.0-W/kg
(i.e., associated with a calorimetry measured value of 2.8- W/kg)
Under the conditions described above using a transmit/receive RF body coil the CORFLO Enteral Feeding Tube produced the Highest temperature change of +1.5 degree celcius during MRI performed for 15-min in the 3-Tesla (3-Tesla/ 128-MHz, Excite, Software G3.0-052B, General Electric Healthcare, Milwaukee, WI) MR system.
Artifact Information: MR image quality may be compromised if the area of interest is relatively close to the position of the CORFLO Enteral Feeding Tube. Therefore, optimization of MR imaging parameters to compensate for the presence of this device may be necessary.
Avanos 40-5432 CORFLO Nasogastric/Nasointestinal Feeding Tube Specifications
| Diameter (Fr) | 12 |
| Catheter Length (In.) | 43 |
| Tube Length (cm) | 91 |
| Tube Length Color Code | Pink |
| Primary Product Color | Yellow |
| Product Brand | CORFLO |
Avanos CORFLO Nasogastric/Nasointestinal Feeding Tube with ENFit Connector 40-5432 Device Characteristics
| What MRI safety information does the labeling contain? | MR Conditional |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as ""Not made with natural rubber latex"": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | No |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |

Avanos Medical #40-4432, CORFLO Nasogastric/Nasointestinal Feeding Tube with Stylet with ENFit Connector, 12FR, 43 in Catherter Length, 91 cm Tube Length, 10 EA/CS
$341.72 per CASE

Avanos Medical #20-9432AIV2, CORFLO Nasogastric/Nasointestinal Feeding Tube with ANTI-IV Connector, 12FR, Catherter Length 43 in, Tube Length 91 cm, 10 EA/CS
$345.35 per CASE

Avanos Medical #40-5438, CORFLO Nasogastric/Nasointestinal Feeding Tube with ENFit Connector, 8FR, 43 in Catherter Length, 91 cm Tube Length, 10 EA/CS
$243.56 per CASE

Avanos Medical #40-1432, CORFLO Nasogastric/Nasointestinal Feeding Tube with ENFit Connector, 12FR, 43 in Catherter Length, 122 cm Tube Length, 10 EA/CS
$167.24 per CASE