-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Avanos Medical FTL8.0P-EO - Orange Polyurethane Oral/Enteral Feeding Tubes, 8.0FR, 90 cm Length, 10 EA/CS
Avanos FTL8.0P-EO Orange Polyurethane Oral/Enteral Feeding Tube
This product is intended for use in neonatal and pediatric patients to provide nutrition via nasal or oral gastric placement and is not intended for use beyond 30 days.
The NeoMed feeding tube is not intended for individual use longer than 30 days, or in patients with congenital anomalies of the GI tract above the stomach requiring surgical intervention. Follow the hospital feeding tube replacement and maintenance procedures or current ADA recommendations as applicable.
- Soft medical grade polymers support nutritive sucking, developmental care and improved outcomes.
- A cost-effective solution providing indwell time up to 30 days, minimizing the need for additional tube placements.
- The semi-encapsulated radiopaque stripe and bold insertion markings to at least 32 cm help confirm placement.
- Not made with natural rubber latex, DEHP, or BPA
- Plugged-style hub closures lend to less bacterial growth than hub closures designed without the ability to plug the lumen or clean hidden reservoirs, threads, caps, or ridges.
- Poor feeding tube hub and hub closure designs including non-swabbable, non-cleanable threaded or recessed hubs and cap closures that do not plug the lumen may lead to material pooling that allows residual EBM exposure to air and potential bacteria colonization to occur in less than 4 hours.
- Contaminated feeding tubes offer a pathway to the gut compromising the health of the gastrointestinal system.
- Both NeoMeds legacy Feeding Tubes and NeoConnect Feeding Tubes with ENFit connectors have plugged closures
Distal Tips
- Our Polyurethane Feeding Tubes have open distal tips without sharp edges or hidden cavities. Large cut holes can allow tissue migration into the port holes, resulting in broken distal tips or torn/plugged tissue upon removal.
- Our feeding tube holes are precision drilled in, instead of cut.
Advance Features of Avanos Orange Polyurethane Oral/Enteral Feeding Tube
 | 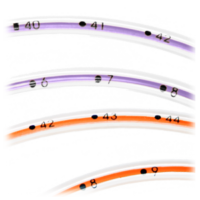 |  |  |
Open distal tip with no sharp edges or hidden cavities helps eliminates residual colonization. | Radiopaque stripe and bold insertion markings at each centimeter help confirm placement | Three offset side portholes help reduce residual colonization | NeoMed's hub and plugged cap are accessible and easily cleaned. Non-swabbable recessed or threaded hubs and caps cause pooling that may increase the risk of bacterial growth. |
Contraindications
The NeoMed feeding tube is not intended for individual use longer than 30 days, or in patients with congenital anomalies of the GI tract above the stomach requiring surgical intervention. Follow the hospital feeding tube replacement and maintenance procedures or current ADA recommendations as applicable.
Warnings/Caution of Avanos Orange Polyurethane Oral/Enteral Feeding Tube
- Do not use if package has been previously opened or damaged.
- Prior to use read entire instructions for use. Failure to do so may result in severe patient injury or death.
- NeoMed feeding tubes are fragile and must be handled with care. Forceps, clamps, and sharp instruments may damage device.
- Do not surgically implant NeoMed nasogastric / orogastric feeding tubes.
- Do not use with a stylet or guidewire.
- Do not separate the hub components from the feeding tube.
- Do not autoclave or re-use feeding tube, as this may increase risk of breakage and biological infection.
- The infants age, clinical conditions, and ability to feed orally will determine the method of feeding.
- Trained personnel well versed in anatomical landmarks, safe technique, and potential complications must perform the procedure.
Use Clean Technique
- Insertion Measurement: measure from the tip of the nose to the earlobe and then from the earlobe to midway between the xyphoid process and umbilicus. Centimeter markings are provided on the catheter to assist in the feeding tube placement.
- A strip of sterile tape can be placed at the target insertion depth of the feeding tube for reference.
- Insert the tube slowly and cautiously into the mouth or nose (lubricant is optional); allow the infant to swallow as the tube is advanced.
- Continue to advance to the predetermined length. Precautions: avoid inadvertent placement of the tube into the trachea or inadequate insertion such that the proximal side hole is not fully placed in the stomach.
- Secure the feeding tube either by holding the tube or taping to the patient before checking for placement.
- Verify feeding tube placement:
- Inject 1cc 2cc of air while auscultating over stomach with stethoscope and listen for air entry.
- Aspirate back 1cc 2cc of air and check for residual gastric content.
- Finalize securement and verify by radiography, as desired. If the tip placement remains uncertain reposition or remove.
- Document the procedure according to hospital procedures and/or policies.
Maintenance of Avanos Orange Polyurethane Oral/Enteral Feeding Tube
- The feeding tube should be checked for correct position and visibility of tube marking each time the tube is used.
- Auscultate to ensure proper tube placement before each use.
- Routinely inspect patients nostril and mouth for tolerating feeding tube position.
- Flush feeding tube with sterile water before and after use.
- Keep feeding tube closed with tethered closure component when tube is not in use
Feeding Tube Removal
- Flush the feeding tube with 0.5cc 1cc of sterile water.
- Close the tethered closure component and/or clamp the feeding tube during tube removal to minimize the risk of aspirating fluids into the trachea during withdraw.
- Gently and slowly withdraw the feeding tube and discard per standard procedures.
- Document the feeding tube removal.
Avanos FTL8.0P-EO Orange Polyurethane Oral/Enteral Feeding Tubes Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |

Avanos Medical #FTS8.0P-EO, Orange Polyurethane Oral/Enteral Feeding Tubes, 8.0FR, 40 cm Length, 10 EA/CS
$160.34 per BOX

Avanos Medical #FTM8.0P-EO, Orange Polyurethane Oral/Enteral Feeding Tubes, 8.0FR, 60 cm Length, 10 EA/CS
$160.34 per BOX

Avanos Medical #FTL5.0P-EO, Orange Polyurethane Oral/Enteral Feeding Tubes, 5.0FR, 90 cm Length, 10 EA/CS
$173.96 per BOX

Avanos Medical #FTL6.5P-EO, Orange Polyurethane Oral/Enteral Feeding Tubes, 6.5FR, 90 cm Length, 10 EA/CS
$173.96 per BOX


