-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Avanos Medical FTM5.0V-EO - NeoMed PVC Enteral Feeding Tube, Legacy, Orange, 5.0 Fr, Length 60 cm, 25/Case
Avanos FTM5.0V-EO NeoMed PVC Feeding Tube
NeoMed offers a variety of feeding tube products using silicone, polyurethane, or PVC materials. NeoMed offers a variety of feeding tube products using silicone (SIL), polyurethane (PUR), or PVC materials.
Polyurethane & silicone feeding tubes provide indwell time up to 30 days, minimizing need for additional tube placements
Advance Features of Avanos FTM5.0V-EO NeoMed PVC Enteral Feeding Tube
 | 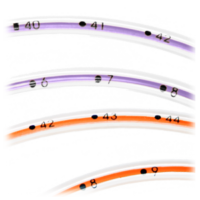 |
Open distal tip with no sharp edges or hidden cavities helps eliminates residual colonization | Radiopaque stripe and bold insertion markings at each centimeter help confirm placement |
 |   |
Three offset side portholes help reduce residual colonization | NeoMeds hub and plugged cap are accessible and easily cleaned. Non-swabbable recessed or threaded hubs and caps cause pooling that may increase the risk of bacterial growth. |
Avanos FTM5.0V-EO NeoMed PVC Enteral Feeding Tube Indications for Use
This product is intended for use in neonatal and pediatric patients to provide nutrition via nasal or oral gastric placement and is not intended for use beyond 30 days.
Avanos FTM5.0V-EO NeoMed PVC Enteral Feeding Tube Contraindications
The NeoMed feeding tube is not intended for individual use longer than 30 days, or in patients with congenital anomalies of the GI tract above the stomach requiring surgical intervention. Follow the hospital feeding tube replacement and maintenance procedures or current ADA recommendations as applicable.
Avanos FTM5.0V-EO NeoMed PVC Enteral Feeding Tube Warnings / Cautions
- Do not use if package has been previously opened or damaged.
- Prior to use read entire instructions for use. Failure to do so may result in severe patient injury or death.
- NeoMed feeding tubes are fragile and must be handled with care. Forceps, clamps, and sharp instruments may damage device.
- Do not surgically implant NeoMed nasogastric / orogastric feeding tubes.
- Do not use with a stylet or guidewire.
- Do not separate the hub components from the feeding tube.
- Do not autoclave or re-use feeding tube, as this may increase risk of breakage and biological infection.
- The infants age, clinical conditions, and ability to feed orally will determine the method of feeding.
- Trained personnel well versed in anatomical landmarks, safe technique, and potential complications must perform the procedure.
Avanos FTM5.0V-EO NeoMed PVC Enteral Feeding Tube Device Characteristic
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |

Avanos Medical #FTM6.5V-EO, NeoMed PVC Enteral Feeding Tube, Legacy, Orange, 6.5 Fr, Length 60 cm, 25/Case
$222.00 per BOX

Avanos Medical #FTM8.0V-EO, NeoMed PVC Enteral Feeding Tube, Legacy, Orange, 8.0 Fr, Length 60 cm, 25/Case
$222.00 per BOX

Avanos Medical #FTS5.0V-EO, NeoMed PVC Enteral Feeding Tube, Legacy, Orange, 5.0 Fr, Length 40 cm, 25/Case
$222.00 per BOX

Avanos Medical #PFTM5.0V-EO, NeoMed PVC Enteral Feeding Tube, Legacy, Purple, 5.0 Fr, Length 60 cm, 25/Case
$222.00 per CASE

