-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Avanos Medical FTS5.0S-NC - Silicone Enteral Feeding Tube with ENFit Connector, Radiopaque Stripe, 5.0 Fr, 40CM Length, 10 EA/CS
Avanos FTS5.0S-NC NeoConnect Silicone Enteral Feeding Tube with ENFit Connector
This product is intended for use in neonatal and pediatric patients to provide nutrition via nasal or oral gastric placement and is not intended for use beyond 30 days.
The NeoMed feeding tube is not intended for individual use longer than 30 days, or in patients with congenital anomalies of the GI tract above the stomach requiring surgical intervention. Follow the hospital feeding tube replacement and maintenance procedures or current ADA recommendations as applicable.
- Soft medical grade silicone supports nutritive and reduces the risk of nipple aversion; this allows for earlier patient discharge and thus lower healthcare costs
- Cost effective solution providing indwell time up to 30 days, minimizing the need for additional tube placements
- Semi-encapsulated radiopaque stripe and bold insertion markings to at least 32 cm help confirm placement.
- No DEHP, no BPA; nonthreaded hub features less bacteria growth as compared to products with hidden reservoirs, threads, caps or ridges
- Feature open distal tips without sharp edges or hidden cavities that allow for complete drainage to reduce opportunity for bacteria growth; large cut holes can allow tissue migration into the port holes, resulting in broken distal tips or torn/plugged tissue upon removal; NeoMed feeding tube holes are precision drilled in, instead of cut
NeoConnect Feeding Tubes
- Open hub design allows visualization and helps mitigate accumulation of HBM and formula that can foster bacterial growth
- Recess-free plug design with wave pattern enhances compliance with cleaning protocols, allows airflow through the hub, and helps reduce bacterial growth1
- Open distal tip (PUR and SIL) with three offset side port holes to help reduce residual colonization associated with closed distal tip feeding tubes
- Securely tethered closure helps reduce risk of separation from feeding tube hub
Advance Features of Avanos NeoConnect Silicone Enteral Feeding Tube with ENFit Connector
 | 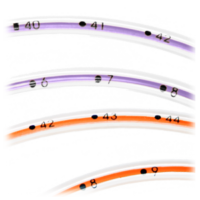 |  |
Open distal tip with no sharp edges or hidden cavities helps eliminates residual colonization. | Radiopaque stripe and bold insertion markings at each centimeter help confirm placement | Three offset side portholes help reduce residual colonization |
 |  |  |  |
NeoConnects open hub design and the associated cleaning tool assist clinicians in the care and maintenance of ENFit feeding tube hubs and their threaded connectors. | Bottomless design allows visualization and eliminates a floor where HBM/formula can accumulate, fostering bacterial growth. | NeoMed offers a plug closure designed to protect the feeding tube opening from hub debris migration that may occur with other "cap" closure systems. | Wave pattern allows airflow through the hub, which helps reduce bacterial growth |
Avanos FTS5.0S-NC NeoConnect Orange Silicone Oral/Enteral Feeding Tubes Device Characteristic
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |

Avanos Medical #PFTS5.0S-NC, Purple Silicone Enteral Feeding Tube with ENFit Connector, Radiopaque Stripe, 5.0 Fr, 40CM Length, 10 EA/CS
$302.94 per CASE

Avanos Medical #FTS6.5S-NC, Silicone Enteral Feeding Tube with ENFit Connector, Radiopaque Stripe, 6.5 Fr, 40CM Length, 10 EA/CS
$301.41 per CASE

Avanos Medical #FTS8.0S-NC, Silicone Enteral Feeding Tube with ENFit Connector, Radiopaque Stripe, 8.0 Fr, 40CM Length, 10 EA/CS
$307.53 per CASE

Avanos Medical #PFTS6.5S-NC, Purple Silicone Enteral Feeding Tube with ENFit Connector, Radiopaque Stripe, 6.5 Fr, 40CM Length, 10 EA/CS
$299.88 per CASE

