-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

B Braun 4251628-01 - CATHETER, IV, INTROCAN, 22GX1", POLY SAFETY, 200/CS

Introcan Safety IV Catheter 22 Ga. x 1 in., PUR, Straight
Introcan Safety Shielded IV catheter without injection port
- Passive fully automatic protection against needlestick injuries and related infections
- IV catheter material available in Polyurethane (PUR) and FEP
- Ergonomic product design for one-handed insertion
- Sharp Universal Bevel for wide choice in insertion angles and minimal puncture trauma
- Double Flashback Technology provides confirmation of successful catheter placement through quick visualization of both needle and catheter flashback
- Latex-free, PVC-free, DEHP-free
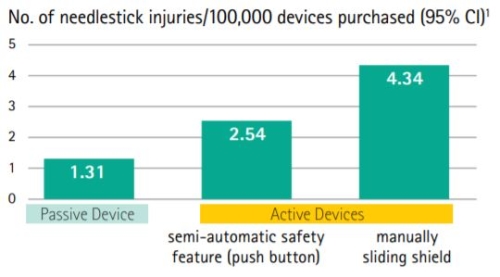
Passive Safety is the best at preventing needlestick injuries
- Fully automatic Passive Safety protection
- Designed for ease of insertion for deep and superficial veins
- Two ways to confirm vein entry
- Environmentally conscious design
- Broad product portfolio including longer length catheters
Every Product Detail Is Designed For Best Practice:
Passive Safety Technology is incorporated into the Introcan Safety IV Catheter via an integrated fully automatic Safety Shield which protects the needle tip to prevent needlestick injuries. A recent study confirmed that passive safety engineered devices create significantly better protection for healthcare workers than those that require the user to activate the safety feature.6 In fact, passive safety devices were associated with the lowest needlestick injury rate and are most effective for needlestick injury prevention.
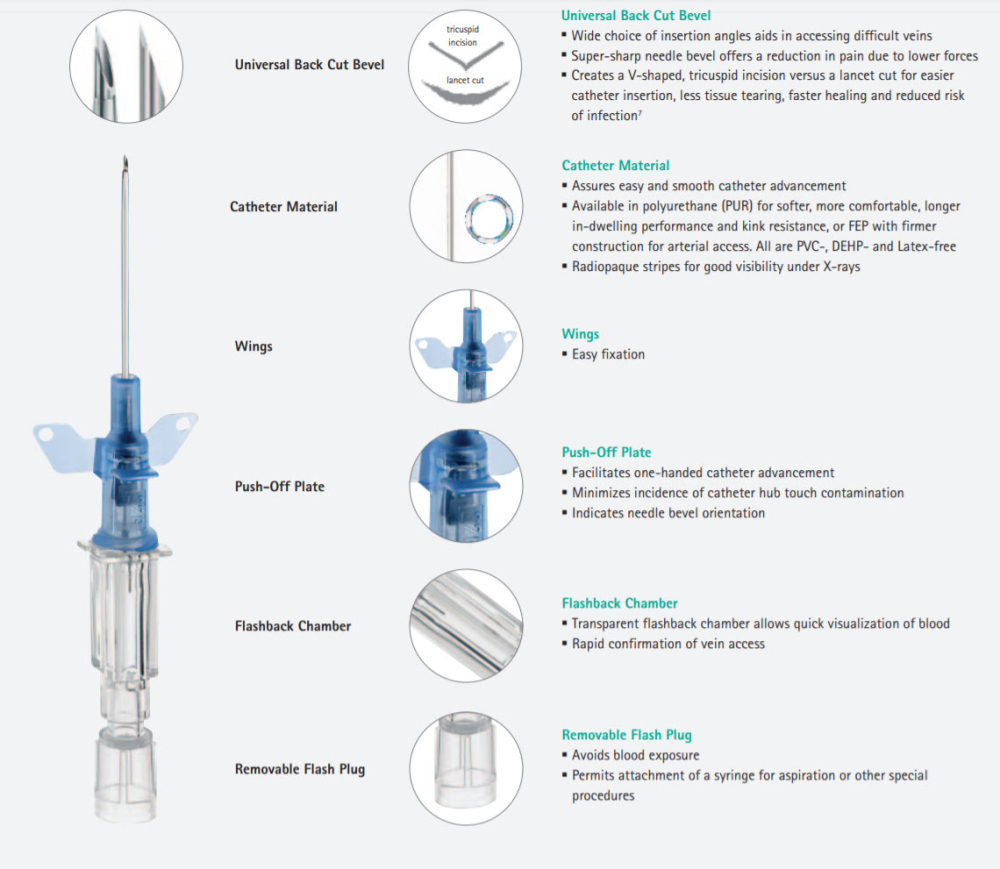
Passive Safety Technology Is Preferred
The Introcan Safety IV Catheters safety mechanism deploys without user activation. Unnecessary injuries may occur with active safety devices because activation is often delayed or never happens. Thats why we designed the B. Braun Introcan Safety IV Catheter and why its raising the standard in needlestick prevention.
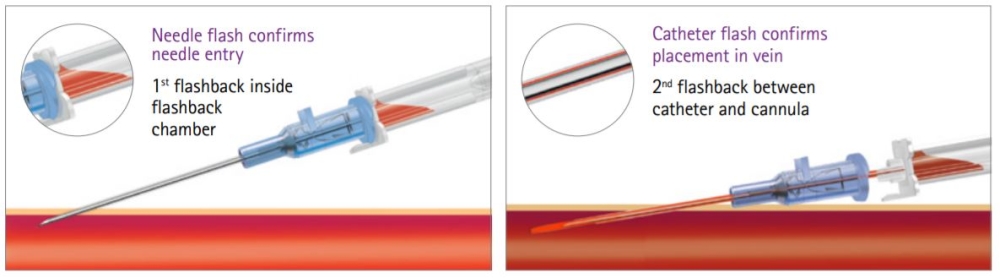
Designed To Promote First-Stick Success
Double Flashback Technology is designed to provide a quick visual confirmation of vein puncture with separate needle and catheter flashback.
Designed To Enable Longer In-Dwelling
Catheters are available in polyurethane (PUR) which provides softer in-dwelling performance,3 or fluorinated ethylene propylene (FEP) with firmer construction for arterial access. Introcan Safety IV Catheters are not made with DEHP or natural rubber latex.
Removable Flashplug
The removable vented flashplug permits attachment of a syringe.

Introcan Safety IV Catheters Help You Cut Costs and "Go Green"
- Generate less waste with smaller, lighter components
- Designed to save money and avoid throwing away unused components
(compared to integrated catheters) - Helps to cut costs by reducing needlesticks, materials, and waste
Introcan Safety IV Catheter - Insertion Guide:
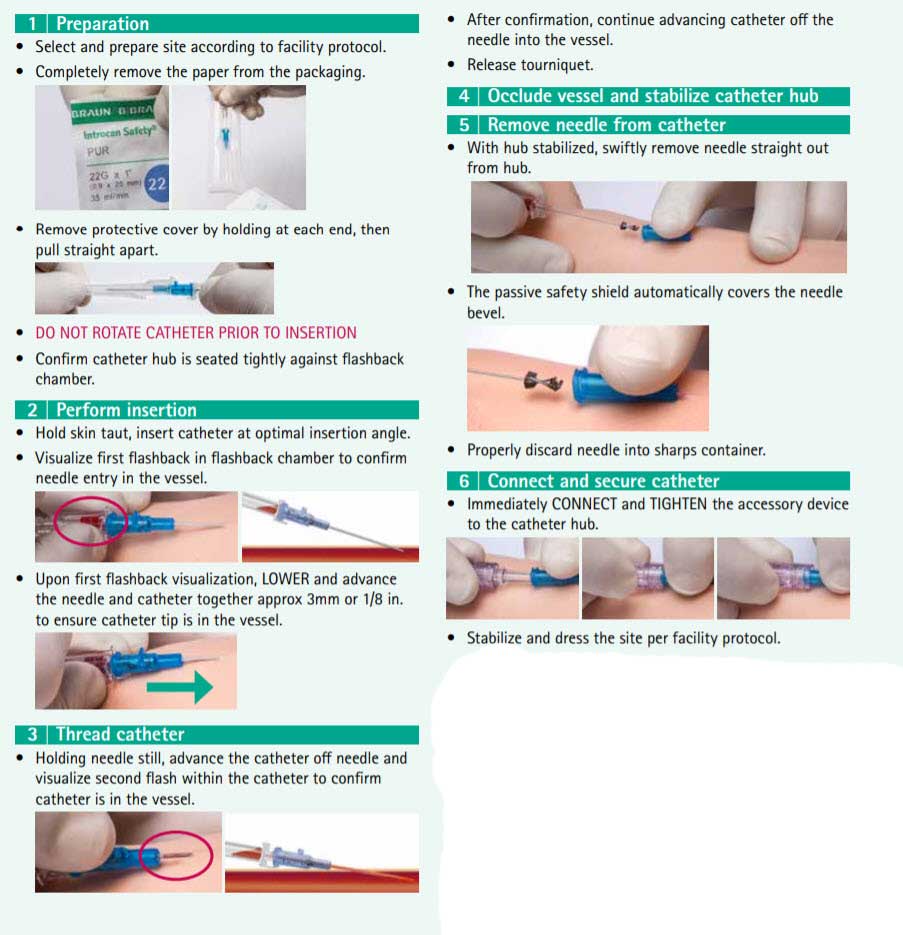
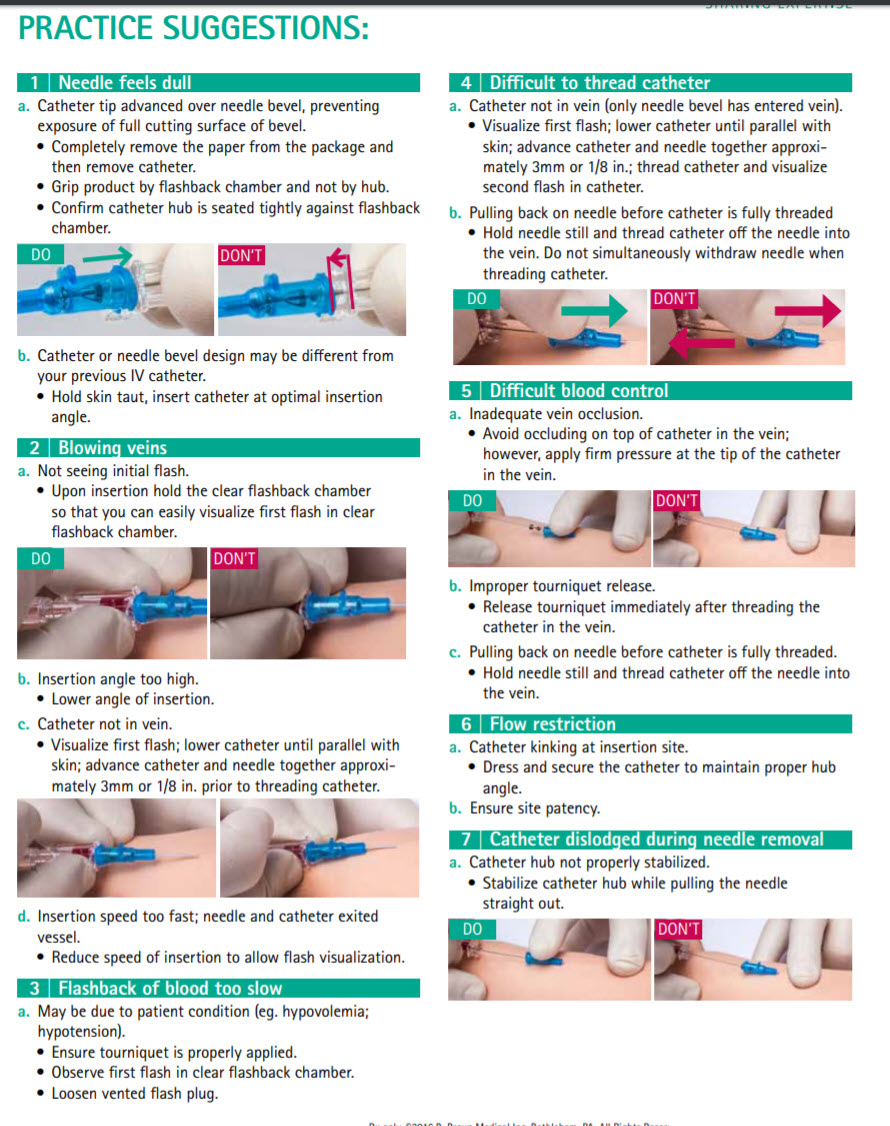
Introcan Safety IV Catheter - Practice Suggestions: