-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

B Braun S8004-5384 - Injection Solution Sod Cl.9% 50mL Fill Non-DEHP/Non-PVC Ea, 84 EA/CA
B Braun S8004-5384 0.9% Sodium Chloride Injection USP, 100 mL Fill in 50 mL PAB
Sodium Chloride Injections USP are sterile, nonpyrogenic, isotonic and contain no bacteriostatic or antimicrobial agents. These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes and water for hydration.
Each mL of 0.9% Sodium Chloride Injection USP contains:
Sodium Chloride USP 9 mg; Water for Injection USP qs pH adjusted with Hydrochloric Acid NF pH: 5.5 (4.57.0) Calculated Osmolarity: 310 mOsmol/liter Concentration of Electrolytes (mEq/100 mL): Sodium 15.4 Chloride 15.4.This solution is sterile, nonpyrogenic, isotonic and contains no bacteriostatic or antimicrobial agents.

The plastic container is a copolymer of ethylene and propylene formulated and developed for parenteral drugs. The copolymer contains no plasticizers and exhibits virtually no leachability. The safety of the plastic container has been confirmed by biological evaluation procedures. The material passes Class VI testing as specified in the U.S. Pharmacopeia for Biological Tests Plastic Containers. These tests have shown that the container is nontoxic and biologically inert.
The container/solution unit is a closed system and is not dependent upon entry of external air during administration. The container has two ports, one is for the intravenous administration set and the other is a medication addition site. Each is covered by a tamperproof barrier. Refer to the Directions for Us e of the container to properly identify the ports.
Clinical Pharmacology
0.9% Sodium Chloride Injection USP provides electrolytes and is a source of water for hydration. It is capable of inducing diuresis depending on the clinical condition of the patient. Sodium, the major cation of the extracellular fluid, functions primarily in the control of water distribution, fluid balance, and osmotic pressure of body fluids. Sodium is also associated with chloride and bicarbonate in the regulation of the acid-base equilibrium of body fluid. Chloride, the major extracellular anion, closely follows the metabolism of sodium, and changes in the acid-base balance of the body are reflected by changes in the chloride concentration.
Indications and Usage
This intravenous solution is indicated for use in adults and pediatric patients as a source of electrolytes and water for hydration. This product is designed for use as a diluent and delivery system for intermittent intravenous administration of compatible drug additives. Consult prescribing information for INDICATIONS AND USAGE of drug additives to be administered in this manner.
PAB Container

PAB IV Container FAQ
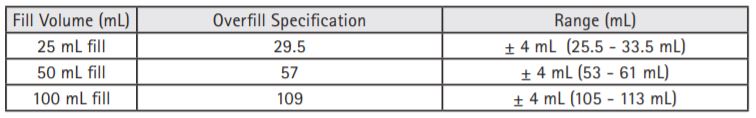
What are the overfill volume specifications for PAB IV containers?
The overfill is required to compensate for the tolerance of the filling equipment, for the volume remaining in the container after use, and for water vapor transmission from the container. The overfill ensures the required volume of solution is present in the container and the concentration remains within specifications through the shelf life of the product. The over-filling specifications for our PAB containers are as follows:
Can PAB IV containers be transported in a pneumatic tube system?
To transport a PAB IV container:
- Fold PAB IV container in half so that the hanger tab is positioned on top and extends past ports to cushion the ports.
- Load PAB IV container into the pneumatic tube carrier in the folded half position.
- Ensure the carrier is securely latched; watch for pinch points.
To transport a PAB IV container with add ease:
- Gently fold PAB diagonally; use caution not to place extra pressure on the bag to avoid premature activation.
- Keeping the diagonal fold on the PAB, position the connection in the pneumatic tube carrier with the vial facing down.
- Close carrier carefully to ensure there are no pinched sides. Close latches.
- B. Braun has not conducted any formal studies to determine the likelihood of inadvertent activation of the addEASE/ PAB system in pneumatic tubes. However, minimizing the application of external pressure reduces the risk of inadvertent activation of the addEASE/PAB container.
Is the port system on the PAB IV container sterile?
The fluid path through the set port is sterile; however, the outside of the port cap (or the outside of the container) is not sterile. The medication port is also not sterile and should be disinfected prior to medication additions. Follow strict aseptic technique during mixing of medications and while attaching a set.



