-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Baxter 2N8399 - LUER ACTIVATED VALVE FOR, 200/CS
Clearlink System Luer Activated Valve for IV Access, Vol. 0.25 mL
Luer Activated Valve for IV Access
CLEARLINK Luer Activated Valve. Approximate volume 0.25 mL. Non-DEHP. Non-PVC. Sterile Package.

Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
Sterilization
| Device Packaged as Sterile: | Yes |
| Requires Sterilization Prior to Use: | No |
MicroClave and CLEARLINK Comparative Matrix
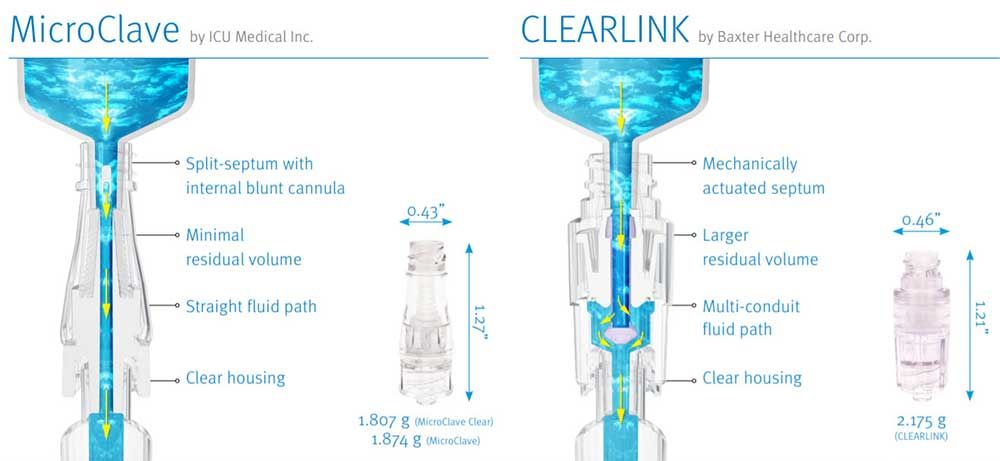
| PRODUCT PERFORMANCE | MICROCLAVE TECHNOLOGY | CLEARLINK TECHNOLOGY |
| Base Technology | Internal cannula and silicone compression seal split-septum. Internal cannula windows are exposed by the insertion of a male luer, and cannula enters the male luers internal space to achieve flow. | Mechanically actuated silicone septum. Insertion of a male luer pushes a silicone membrane apart and an internal poppet down. Fluid flows from the luer into the internal poppet, through the poppet windows, and around a fluid director into the lower connector housing and into the catheter hub. |
| Displacement | Neutral: 0 to -0.01 mL | Negative: 0.13 mL |
| Residual Volume | 0.04 mL | 0.25 mL (over 6 times larger |
| Fluid Path | Straight through polycarbonate cannula. Enhances flushing efficiency | Fluid exits male luer into a polycarbonate poppet, out window into lower housing. |
| Moving Parts in Fluid Path | No | Yes |
| Number of Assembly Parts | 3, of which 1 moves on luer access. | 4, of which 2 move on luer access. |
| Fluid Residual External on Disconnect | Minimal | Minimal |
| Clamping Sequence | None required | Yes, clamp before disconnect. |
| Flow Rate | 165 mL/min | 118 mL /min |
| Clear Available | Yes | Yes |
| Antimicrobial Available | Yes | Yes |
| Patient Comfort | 7% smaller profile, 14-17% less weight. Smooth profile. | Larger and heavier than MicroClave. |
| Bacterial Transfer Performance | The least amount of bacterial transfer of any connector tested. | Exhibits a higher bacterial transfer rate than MicroClave. |
| Flushing Performance | Highly efficient. Connector clear of blood elements with minimal flush volumes from 2 to 7.5 mL. Not recommended to change connector after blood draw. | Baxter recommends flushing CLEARLINK connector with 10 mL or more after blood infusion/sampling. If CLEARLINK connector cannot be cleared of blood after blood infusion/sampling, replace immediately. |
Product Characteristics
| Priming Volume | 0.25 ML |
| Type of Injection Site | CLEARLINK |
| Power Injectable | No |
| Sterility | Sterile Package |
| Rx Only | Yes |
Packing Information
| Length | 7 IN |
| Width | 6.7 IN |
| Height | 6.1 IN |
| Volume | 0.17 FC |
| Weight | 2.2 LB |
| Pack Factor | 200 |

Baxter #2N8399, VALVE, CLEARLINK, LUER ACTIVATED, FOR IV, EACH
$4.77 per EACH

Baxter #2N8399, Clearlink Luer Activated Valve, 0.25 ml, Non-DEHP, Non-PVC, Sterile, 200/cs (Rx) (Continental US Only, Excluding IN and ND) (Product Access Restricted. Check with your sales rep to verify eligibility)
$955.31 per CASE

Baxter #2N8334, CONNECTOR-ADAPTER IV TRI PORT MALE LUER LOCK DEHP-FREE STERILE (200/CS)
$1,996.71 per CASE

Baxter #2N8341, Catheter Extension Set, 3-Lead, Microbore, Clearlink Luer Activated Valve, Male Luer Lock Adapter, 0.82 ml, 5.5" Length, Non-Pyrogenic, Non-DEHP, Sterile, 200/cs (Rx) (Continental US Only, Excluding IN and ND)
$2,118.29 per CASE

