-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

BD 328440 - Syringe .3cc 31gx5/16" U100 Ultrafine Insulin Short Bevel 100/Bx, 5 BX/CA
BD Ultra-Fine Short Needle Half Unit Insulin Syringes 3/10mL 8mm (5/16") 31G
328440 - BD Insulin Syringes with the BD Ultra-Fine needle 8mm x 31G 3/10 mL/cc Half unit scale
BD 328440 Ultra-Fine Needle is a 31-gauge, 8mm (5/16") syringe needle engineered to provide comfort, accuracy and ease in injecting. Reduces the risk of a painful injection in the muscle.
- Greater Efficacy: Efficacy equivalent to longer needles
- Patient Choice: Preffered by over 80% patients
- Less intimidating: Less intimidating for patients
- Reduced Risk: Reduces the risk of intramuscular injection
- Self-contained insulin syringe with permanent needle for use with U-100 insulin.
- Packaging contains end user directions and information.
Key Product Features
| Hub Color | Clear |
| Hub Material | Polypropylene |
| Hub Type | Rounded |
| Needle Gauge | 30 G |
| Needle Gauge (m) | 0.30 mm |
| Needle Length (in.) | 5/16 in. |
| Needle Length (m) | 8 mm |
| Needle Tip Type | 3-bevel |
| Needle Type | Insulin |
| Needle Wall Type | Regular |
| Syringe Scale | 0.3 mL graduations |
| Sterile | Sterilized product |
| Safety Engineered | Safety engineered product |
| Safety Engineered Feature | Hypodermic syringe and needle with shielding mechanism |
| Sterilization Method | Gamma radiation |
| Latex Statement | Not made with natural rubber latex |
| Disposable | Disposable product |
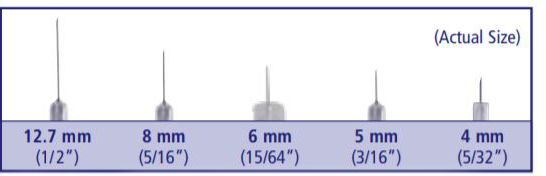
Needle length (mm)
Insulin needles come in different lengths. BD Original Needles are 12.7 mm (1/2") long, and BD Short Needles are 8 mm (5/16") long. The BD Nano Needle 4mm (3/16") and BD Mini Needle 5mm (5/32") are available only in pen needles. For a comfortable injection experience, shorter needle lengths are recommended and preferred.
Needle gauge (G)
The higher the gauge, the thinner the needle. For example, 32G is thinner than a 31G needle.Insulin needles are available in many gauges (G), or thicknesses. The higher the gauge, the thinner the needle. For example, a 32 G needle is actually thinner and more comfortable than a 29 G needle.
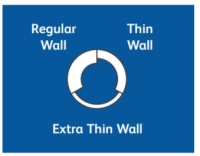
Regular Wall
Regular Wall:This is the most common wall thickness. The thickness of the steel wall allows a good flow rate, and minimizes flexing when the needle is inserted into a vial stopper or patient.
Permanently Attached Needle
Most commonly found in insulin and 'tuberculin' syringes. Permanently attached needles, also known as integral needles, reduce the amount of medication waste and allow accurate mixing of different medications into one syringe.
Regulatory Compliance and Quality System
BD Products comply with the regulatory requirements of the region in which these are sold and manufactured.
Sterility
All products which are labeled as #sterile# and released for sale by BD are certified to be sterile per EN 556-1 Sterilization of Medical Devices as long as the package is unopened and undamaged. This product is sterilized via Cobalt 60 - Irradiation. Sterilization cycle development/validation is performed to 10-6 SAL in accordance with current ISO 11137 guidelines.
Biocompatability
This product has been evaluated in accordance with ISO 10993 "Biological Evaluation of Medical Devices", and complies with all relevant sections.
Pyrogenicity
All products which are labeled as non-pyrogenic and released for sale by BD have been tested per United States Pharmacopeia (USP) chapter (85)- Bacterial Endotoxins Test and meets limits as specified in chapter 161- Transfusion and Infusion Assemblies and Similar Medical Devices.
Quality Control Testing and Release
Representative production samples are collected and inspected in accordance with current applicable product specifications. Inspection records are reviewed and signed off by qualified personnel for product release. The released devices meet applicable BD product specification(s).
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437) | No |
| Device labeled as "Not made with natural rubber latex" | No |
| For Single-Use | Yes |
| Prescription Use (Rx) | No |
| Over the Counter (OTC) | No |
| Kit | No |
| Combination Product | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P) | No |
Proper Injection Technique
Proper injection technique is essential to improve consistency in medication delivery and optimize glycemic control. It includes factors such as:
- knowing where the common injection sites are
- understanding how to rotate sites
- ensuring consistent delivery into subcutaneous (fat) layer
- choosing proper needle length
- avoiding injecting into muscle
Why is Proper Disposal Important?
Proper syringe disposal will help to:
- Store and safely dispose of used syringes and lancets,
- Protect trash collectors from accidental needlesticks,
- Prevent your used syringes from falling into the wrong hands,
- Protect the environment.
Product Packaging Information
| Packaging Level | Shelfpack | Case | Each |
| Quantity | 100 | 500 | 1 |
| Length | 171.45 mm | 43.815 cm | |
| Width | 139.7 mm | 18.415 cm | |
| Height | 84.138 mm | 15.24 cm | |
| Weight | 310.0 g | 1.73 kg | 3.636 g |

BD #328468, Syringe .5cc 31gx5/16" U100 Ultrafine Insulin Short Bevel 100/Bx, 5 BX/CA
$392.02 per CASE

BD #328468, Syringe .5cc 31gx5/16" U100 Ultrafine Insulin Short Bevel 100/Bx, 5 BX/CA
$316.67 per CASE

BD #328431, Syringe .3cc 30gx1/2" U100 Ultra-fine Insulin w/ Needle 100/Bx, 5 BX/CA
Call for Pricing

BD #328418, Syringe 1cc 31gx5/16" U100 Ultra-Fine Insulin 100/Bx, 5 BX/CA
$304.33 per CASE