-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

BD 382637 - BD 382637 Insyte Autoguard BC Shielded IV Catheter With Blood Control Technology, Pink, Winged, 50/Box, 4 Box/CS
BD 382637 Insyte Autoguard BC Shielded IV Catheter With Blood Control Technology
The BD Insyte Autoguard BC shielded IV catheter with blood control technology is clinically demonstrated to reduce the risk of blood exposure by 95%. BD blood control technology minimizes blood exposure, cleanup time and use of supplies associated with blood spills.
- Safety IV catheter designed to reduce blood exposure and needlestick injury
- Blood control technology designed to stop blood flow from the catheter hub once, at insertion
Blood leakage prevention septum | Increased safety | First-stick success | Minimized phlebitis |
| You do not need to worry about stopping blood during initial insertion with the BD Insyte Autoguard BC catheter. | BD Insyte Autoguard IV catheters demonstrated a statistically significant 95% reduction in needlesticks. | BD Instaflash needle technology on the 20-G, 22-G and 24-G sizes incorporates a notched needle to improve first-stick success and reduce painful hit-and-miss insertions. | BD Vialon biomaterial softens up to 70% in the vein, enabling longer dwell times3 and reducing the chance of mechanical phlebitis by up to 50% |
| Catalog No. (Non Winged | Winged) | Color | Gauge | Length (in) |
| 382537 | 382637 | Pink | 20 | 1.88 in. |
| Catheter ID (mm) | Catheter OD(mm) | Gravity Flow Rate (mL/min) | High Pressure Rating (psi) |
| 0.7620 - 0.8382 | 1.0414 - 1.1176 | 54 | 300 |
Clean, easy and efficient for clinicians and patients You strive for a clean IV placement every time, but when you have to apply pressure while activating the devices safety mechanism and connecting the extension, blood spills can happen. The BD Insyte Autoguard BC shielded IV catheter with blood control technology addresses these challenges:
| 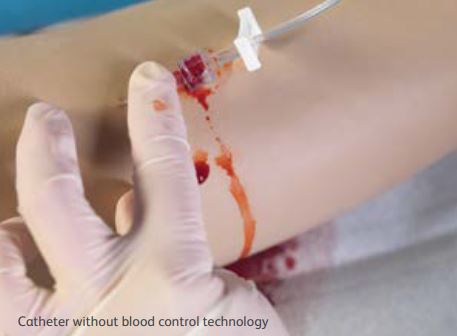 |
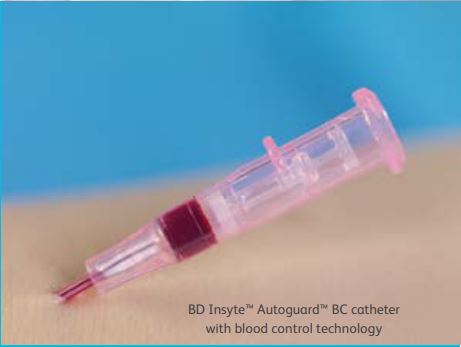 | Built-in blood leakage prevention No need to worry about stopping blood during insertion with BD Insyte Autoguard BC catheters. Stop blood leakage without the need for venous compression - the unique design allows a one-handed insertion technique with blood flow resuming when an add-on device is connected. Clinically demonstrated to reduce the risk of blood exposure by 95% - reducing staff exposure to blood as well as surface contamination. |
Safety at the push of a button The BD Insyte Autoguard BC catheter is tailored for the clinicians safety and insertion success, leaving you feeling confident in each insertion, and your patients feeling confident in you. Demonstrated to reduce needlestick injuries by 95%
|  |
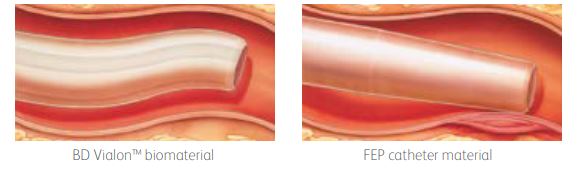 | The right IV catheters, right from the start BD Vialon Biomaterial The catheter material is designed to resist kinking and softens up to 70% in the vein, enabling longer dwell times and reducing the chance of mechanical phlebitis by up to 50%. |
BD 382637 Insyte Autoguard BC Shielded IV Catheter Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |

BD #382534, BD Insyte Autoguard BC Shielded IV Catheter With Blood Control Technology, Pink, Non Winged, 50/Box, 4 Box/CS
Call for Pricing

BD #382534, BD Insyte Autoguard BC Shielded IV Catheter With Blood Control Technology, Pink, Non Winged, 50/Box, 4 Box/CS
$710.12 per CASE

BD #382612, BD Insyte Autoguard BC Shielded IV Catheter With Blood Control Technology, Yellow, Winged, 50/Box, 4 Box/CS
$549.50 per CASE

BD #382623, BD Insyte Autoguard BC Shielded IV Catheter With Blood Control Technology, Blue, Winged, 50/Box, 4 Box/CS
$753.36 per CASE


