-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

BD 383346 - BD Saf-T-Intima Closed IV Catheter System, 18 G x 1.00 in. (1.3 mm x 25 mm), 25/Box, 200/CS
BD 383312 BD Saf-T-Intima closed IV catheter
The BD Saf-T-Intima closed IV catheter system+ incorporates preattached extension tubing to help support reduction in blood exposure.
Features and Benefits of BD 383312 BD Saf-T-Intima closed IV catheter
Advanced shielding design
A telescoping needle shield passively covers the needle, helping protect clinicians from needlestick injuries.
Closed system
Preattached extension tubing offers a closed system to minimize blood exposure during catheter insertion.
Removable adapter
A removable PRN adapter allows the use of an ISO-compliant needleless connector.
BD Vialon biomaterial
BD Vialon - A trusted biomaterial that improves dwell time with less risk of phlebitis4 in IV therapy, has the same properties to ensure optimal subcutaneous dwell times.
The system is constructed with BD Vialon biomaterial, a proprietary catheter material that softens in the vein.
BD Saf-T-Intima Integrated closed Safety IV System now indicated for subcutaneous infusion therapy
The use of products which exhibit these features is recommended by leading consultants
- 24G short peripheral intravenous catheter
- Comfortable wings for support and stabilization
- Integrated short extension set to aid smooth insertion and reduce catheter movement during use
- Integrated safety mechanism to ensure health care worker safety during and after placement of device
The use of intravenous catheter systems for subcutaneous infusion therapy is predominant. Compared to winged steel needle sets, peripheral IV catheters will:
- Dramatically reduce needle stick injuries
- Increase dwell time
- Reduce risks from steel sharp under patients skin
- Reduce skin reactions
The design of the intravenous catheter can aid patient comfort and help ensure optimal dwell times are achieved.
BD 383312 BD Saf-T-Intima closed IV catheter Insertion Guide
Preparation
- Hold as shown (Fig. 1) and rotate the white safety shield to loosen the needle. (Fig. 1).
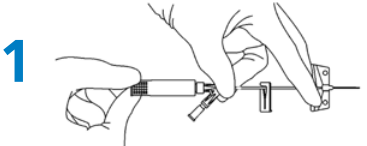
Priming
- To prime the BD Saf-T-Intima Y port: remove the vent plug and prime with fluids according to the organizations procedure. Replace the vent plug after priming, before insertion.
Insertion
- Grasp the textured sides of wings and bring them together, pinching firmly. (Fig. 2A).
- Using thumb and index finger gently pinch the skin around selected site to identify the subcutaneous tissue. (Fig. 2B).
- Insert the full length of the catheter and needle through the skin at a 30℃-45℃ angle. (Fig. 2B).

Needle Removal
- Lay the wings flat on the skin surface and pull the white safety shield in a straight, continuous motion until the safety shield separates from the safety system. (Fig. 3).
- Discard the needle immediately in a puncture resistant sharps container.
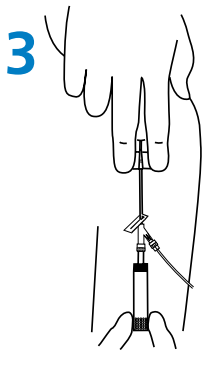 |  |
Stabilization
- Secure the catheter and apply a sterile dressing and end cap according to the organizations procedure.
Device Characteristics of Dale Medical H84101501 Hold-n-Place Foley Catheter Holder
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |

BD #383312, BD Saf-T-Intima Closed IV Catheter System with BD Vialon Biomaterial, 18 G x 1.00 in. (1.3 mm x 25 mm), 25/BOX, 200/CS
$148.30 per BOX

BD #383312, BD Saf-T-Intima Closed IV Catheter System with BD Vialon Biomaterial, 18 G x 1.00 in. (1.3 mm x 25 mm), 200/CS
$796.50 per CASE

BD #383312, BD Saf-T-Intima Closed IV Catheter System with BD Vialon Biomaterial, 18 G x 1.00 in. (1.3 mm x 25 mm), 200/CS
$724.24 per CASE

BD #383312, BD Saf-T-Intima Closed IV Catheter System with BD Vialon Biomaterial, 18 G x 1.00 in. (1.3 mm x 25 mm), 200/CS
$685.46 per CASE

