-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

BD 383591 - BD Nexiva Diffusics Closed IV Catheter System, 22 G x 1.00 IN. 20/Box 80/Case
BD 383591 Nexiva Diffusics Closed IV Catheter System
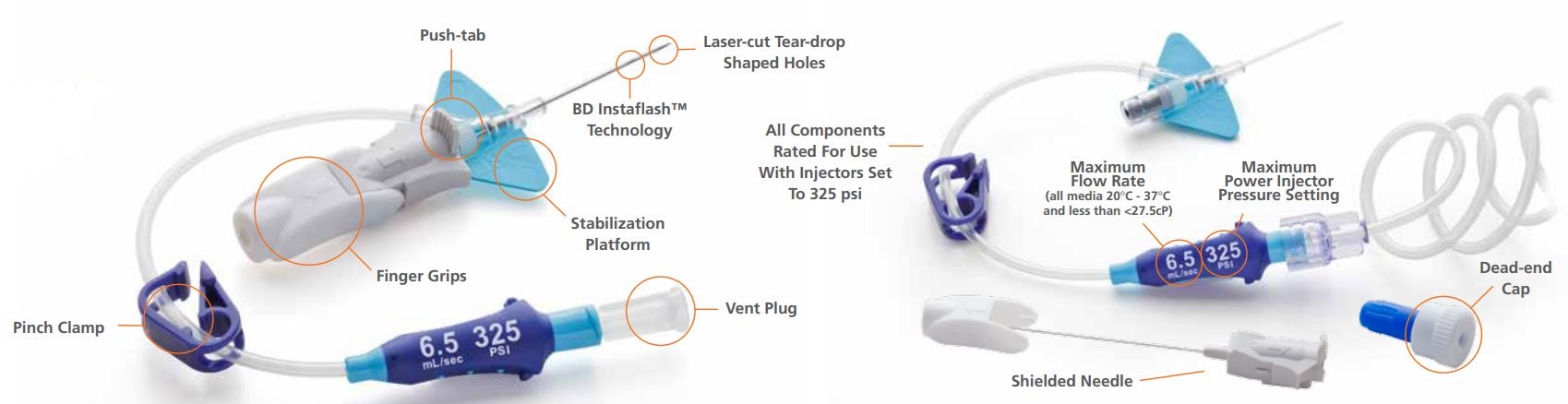
Closed IV catheter system for radiographic power injection
The BD Nexiva Diffusics closed IV catheter system addresses common computed tomography (CT) challenges during power-injection procedures, while helping you efficiently deliver care and minimize the risk of complications.
| Catalog No. | Color | Gauge | Length (in) |
| 383591 | Blue | 22 | 1.00 in. |
| Extension Tube I.D. | Gravity Flow Rate | Max CT Flow Rate | Packaging |
| 1.65 mm | 2690 mL/hr | 6.5 mL/sec | 20/Box 80/Case |
BD 383591 Nexiva Diffusics Closed IV Catheter System Features & Benefits
Built-in design
The BD Nexiva Diffusics system incorporates a built-in stabilisation platform.
Preassembly
The BD Nexiva Diffusics system is a pre-assembled closed IV catheter system.
Luer adapter
The system features a luer adapter with indication of maximum flow rate and pressure setting.
Integrated system
The system features an all-in-one catheter and extension set built for your power injector's 325 psi setting.
Can the BD Nexiva Diffusics closed IV catheter system be used for both general infusions and/or power injection?
Yes. The BD Nexiva Diffusics closed IV catheter system can be used for administration of fluids and power injection of contrast media.
How does this closed IV catheter design meet clinical best practices?
The closed IV catheter is designed to keep blood contained within the device during insertion. It allows for access away from the insertion site, which may minimize irritation to the vessel related to catheter movement. It may be used in conjunction with the specially designed dressing for use with the BD Nexiva closed IV catheter system, which has been shown to provide stabilization,1 and is recommended by both the Infusion Therapy Standards of Practice and the CDC for peripheral IV catheters.
How is the BD Nexiva Diffusics closed IV catheter system different from the BD Nexiva closed IV catheter system?
The BD Nexiva Diffusics closed IV catheter system has three laser-cut, teardrop-shaped holes positioned near the catheter tip and a strengthened design that enables use with power injector set to a maximum pressure of 325 psi. Furthermore, the 24 gauge BD Nexiva Diffusics system is power-injectable.
BD 383591 Nexiva Diffusics Closed IV Catheter System Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |

BD #383591, BD Nexiva Diffusics Closed IV Catheter System, 22 G x 1.00 IN. 20/Box 80/Case
$899.00 per CASE

BD #383594, BD Nexiva Diffusics Closed IV Catheter System, Pink, 20 G x 1.25 IN. 20/Box 80/Case
$191.12 per BOX

BD #383512, BD Nexiva Closed IV Catheter System Single Port, 22 G x 1.00 in. (0.9 mm x 25 mm) Blue, 20/Box 80/Case
$150.29 per BOX

BD #383532, BD Nexiva Closed IV Catheter System Dual Port, 22 G x 1.00 in. (0.9 mm x 25 mm), Blue, 20/Box 80/Case
$227.50 per BOX

