-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

BD 385100 - Q-Syte Luer Access Split-Sept Device 50/BX
BD Q-Syte Luer Access Split-Septum Stand-Alone Device
The BD Q-Syte luer activated split septum is a true split septum device that combines luer access with a split septum valve to provide a closed IV system. As a true split septum device, it conveniently provides a simple fluid path with no internal mechanism and a clear housing that allows you to easily view and assess the fluid path.
- Smooth surface is easily cleaned prior to access.
- No crevices or gaps around the surface to harbour bacteria.
- Clear housing allows visual assessment of the fluid path.
- Simple fluid path design reduces places for microbes to grow;4 this fluid path also delivers a better flow rate.
Key Product Features
| CE Mark | Product is CE-marked |
| Flow Rate Gravity | 32 L/hr |
| Needle Access Type | Luer lock |
| Priming Volume | 0.10 mL |
| Total Shelf Life | 1825 |
| Sterile | Sterilized product |
| Type of Clamp | Slide |
| Safety Engineered | Safety engineered product |
| Safety Engineered Feature | Needleless |
| Sterilization Method | EO |
| DEHP Free | Not made with DEHP |
| Latex Statement | Not made with natural rubber latex |
| PVC Free | Not made with PVC |
| Disposable | Disposable product |
| Single Use | Product is for single use only |
A Split-Septum Needleless Access System Has 64% 70% Lower CRBSI Rates Than Mechanical Valves
The Department of Health has estimated that are 300,000 healthcare associated infections each year. The Health Protection Agency reported that bloodstream infections have increased from 80,000 in 2003 to 105,000 in 2007. The National Audit Office report on reducing Healthcare Associated Infections in June 2009 reported that 44% of bloodstream infections are associated with invasive devices, two thirds of these due to intravenous access devices such as peripheral and central line catheters. Although a small and seemingly inconsequential component of an infusion therapy system, a needleless access device can be the place of origin for microbial growth.
Purposefully simple in design and function, split-septum devices eliminate the complexities of mechanical valves, and with them, the places that may harbour bacteria. In fact, studies comparing devices found that patients are three times more likely, on average, to develop a catheter related blood stream infection (CRBSI) with the use of mechanical valves vs. a split-septum needleless access system.

The Solution is in Simplicity
The split-septum concept was introduced to the needleless IV access device market with Interlink. BD understands that split-septum features such as simple internal design, ease of use, and a straight, clear fluid path, are critical to achieve CRBSI reductions. Now, BD Medical extends the benefits of split septum to the convenience of luer access with BD Q-Syte Luer Access Split Septum.
Ultimate Performance in Closed Luer Access
In addition to the benefits of split-septum design, the BD Q-Syte device delivers optimal luer access performance. Because of its straight and unobstructed fluid path, the BD Q-Syte device provides:
- Dramatically higher flow rates
- U A low priming volume
- U Flexibility to use ISO-compatible luer slip or luer lock connection
Flow Rate Comparison Luer Access Devices
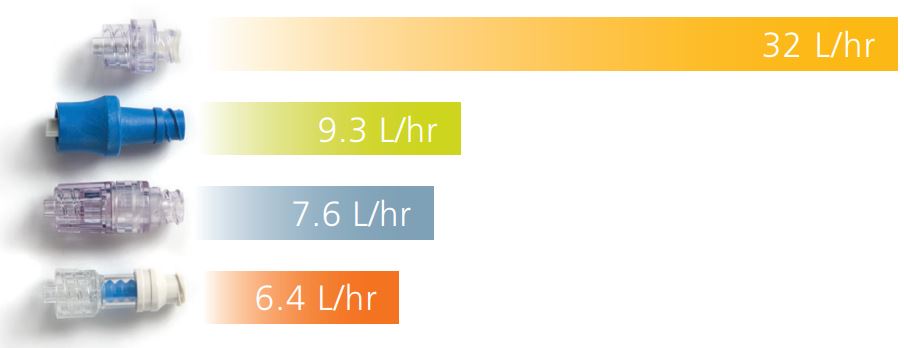
Simple design. Dramatic results.
The Impact of Needleless Connectors on CRBSIs
Recently updated guidelines from the Centers for Disease Control and Prevention (CDC) offer critical, new evidence-based interventions to help you overcome the challenge of catheter related bloodstream infections (CRBSIs). Studies have shown that the rate of these infections may be reduced simply by the type of device clinicians use on their patients. When compared with mechanical valves, split-septum devices have 64%-70% lower CRBSI rates. Furthermore, there has been three Australian publications that demonstrated the introduction of mechanical valve access devices had resulted in a least a doubling of CRBSI rates.
A True Split-Septum by INS Standards
While many valves may claim to be split septum, the split-septum devices described in the evidence cited by the CDC were BD Q-Syte Luer Access Split Septum and Interlink. The 2011 Infusion Nursing Standards of Practice defines a split septum device as a simple needleless connector with a pre pierced septum that can be of blunt cannula or luer-lock design.7 What makes the BD Q-Syte device a true split septum is the simple fl uid path with no internal mechanism. This device eliminates the complexities of mechanical valves, and with them, the places that may harbor bacteria.
"The nurse should be aware that the catheter hub is a known source for the development of CRBSI and that needleless connectors are recognized sites for microbial contamination."
Helping You Meet Best-Practice Standards and Guidelines
BD has a range of products to support your needs to successfully implement critical standards and guidelines from the CDC and INS. It begins with BD Q-Syte, which has shown positive outcomes in peer-reviewed evidence. In addition, we will supply educational materials and conversion support.
Compliance With New Regulations
The Government has made reduction of healthcare associated infections a priority. Previously the Healthcare Commission has monitored infection rates in trusts and aided in their prevention. From 1 April 2009 additional requirements have been published by the newly formed Care Quality Commission. This commission will continue to complete annual inspections of hospitals and will monitor compliance with the Health and Social Care Act 2008 which has published a code of practice to prevent and reduce healthcare associated infections.
Because the cost to the NHS of hospital associated infections is estimated to be in excess of 1 billion per year , taking strides to reduce the risk of CRBSIs is in the best interest of hospitals and patients alike. Utilising split-septum devices such as BD Q-Syte may help hospitals reduce their rate of CRBSIs, an outcome that is good for patients, the healthcare institution and the bottom line.
Product Packaging Information
| Packaging Level | Shelfpack | Case | Each |
| Quantity | 50 | 200 | 1 |
| Length | 127.0 mm | 261.62 mm | |
| Width | 91.94 mm | 200.66 mm | |
| Height | 101.6 mm | 121.92 mm | |
| Weight | 198.446 g | 793.786 g | 3.969 g |

BD #385100, Q-Syte Luer Access Split-Sept Device, EACH
Call for Pricing

BD #385100, DEVICE CLOSED LUER ACCESS QSYTE 200/C
$346.10 per CASE

BD #385100, Q-SITE CLOSED LUER ACCESS DEVICE, 200/CS
$351.00 per CASE

BD #385100, Device Split Septum Q-Style PVC Free Needleless .1 mL Luer 50/Bx, 4 BX/CA
$482.36 per CASE

