-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Cook Medical G02601 - NEEDLE, BREAST, KOPANS MOD, DKBL-20-7.0-A, EACH
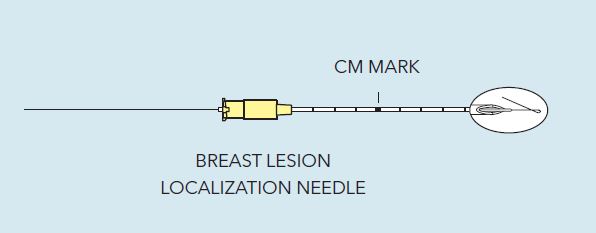
Kopans Breast Lesion Localization Needle
The Kopans Breast Lesion Localization Needle consists of a bevel-tip needle with centimeter markings and a hookwire. The hookwire features a burnish mark that allows the operator to ensure that the hookwire is within the needle tip during needle manipulation.
- Bevel-cut needle tip guides the needle to the optimum position for placing the localization hook wire.
- Reference burnish mark, when visible just outside the needle hub on the spring-hook wire, provides visual assurance that the hook wire is within the needle tip during needle manipulation.
- Needle is marked in 1 cm increments for depth guidance during a procedure.
- The reinforced tip of the spring-hook wire serves as a visual and palpable landmark for the surgeon to gauge distance to the lesion during dissection and reduces the risk of severing the hook wire.
Used for preoperative marking of nonpalpable breast lesions.
| Order Number | Reference Part Number | MR Status | Needle gage | Length cm |
| G02601 | DKBL-20-7.0-A | - | 20 | 7 |
Intended Use
The Kopans Breast Lesion Localization Needle is intended for preoperative marking of nonpalpable breast lesions.
Sterilization
| Device Packaged as Sterile | Yes |
| Requires Sterilization Prior to Use | No |
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
Warnings
Under no circumstances should a hookwire engaged in tissue be pulled out without surgical intervention.
Precautions
- This product is made of ferrous material and has not been tested for safety in the MR enviroment.
- The product is intended for use by physicians trained and experienced in diagnostic and interventional techniques. Standard techniques for localization of breast lesions should be employed.
- Following placement of the hookwire, the portion protruding outside of the breast should be bent and taped to the skin to prevent inadvertent movement.
- Final hookwire position should be confirmed by appropriate imaging modality.
- The hookwire should be used as a guide for the surgeon, not a retractor.
Potential Adverse Events
- Infection
- Internal bleeding and/or bleeding at the puncture site
- Pneumothorax
- Puncture or injury to target organ or to a nearby organ that is traversed by the needle.
- Hemoptysis
- Peritonitis
- Damage of tissue adjacent to the needle
Instructions for Use
NOTE: The burnish mark on the hookwire should be seen just outside the needle hub to ensure that the hookwire is within the needle tip during needle manipulation.
- Introduce the needle into the lesion.
- Check the position of the needle.
- Advance and release the hookwire.
- Remove the needle.
- Bend the hookwire protruding from the breast and tape flat to the skin.
- Verify final hookwire position.
How Supplied
Supplied sterilized by ethylene oxide gas in peel-open packages. Intended for one-time use. Sterile if package is unopened or undamaged. Do not use the product if there is doubt as to whether the product is sterile. Store in a dark, dry, cool place. Avoid extended exposure to light. Upon removal from package, inspect the product to ensure no damage has occurred.

Cook Medical #G02587, NEEDLE, BREAST, KOPANS MOD, DKBL-20-9.0-A, EACH
$130.62 EACH

Cook Medical #G02587, NEEDLE, BREAST, KOPANS MOD, DKBL-20-9.0-A, EACH
$126.36 EACH

Cook Medical #G02587, NEEDLE, BREAST, KOPANS MOD, DKBL-20-9.0-A, EACH
$600.00 EACH