-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Cook Medical G02647 - MRT-9.5-130 MCLEAN-RING ENTERAL FEED, EACH
McLean-Ring Enteral Feeding Tube Set
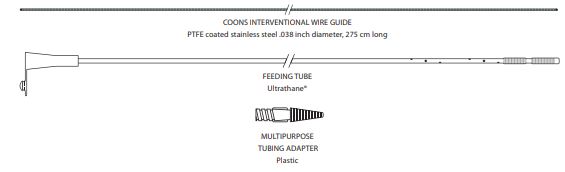
The McLean-Ring Enteral Feeding Tube Set consists of:
- 1 TFE-coated, stainless steel Coons interventional wire guide
- 1 Ultrathane feeding tube
- 1 plastic multipurpose tubing adapter
The feeding tube has a weighted distal end to facilitate passage. The feeding tube endhole allows placement over a wire guide if ineffective peristalsis or obstruction exists.
| Order Number | Reference Part Number | Feeding Tube Fr | Feeding Tube Length (cm) | Feeding Tube | Feeding Tube Tip Configuration | Wire Guide Length (cm) |
| G02647 | MRT-9.5-130 | 9.5 | 130 | 6 | Open end | 275 |
Features and Benefits
- The weighted distal end facilitates the passage of the tube.
- The tube can be placed over a wire guide through the end hole if an obstruction or an ineffective peristalsis exists.
- The tube has an open distal end.
Components
- Coons Interventional Wire Guide
- Feeding tube
Intended Use
The McLean-Ring Enteral Feeding Tube is intended for enteral feeding. The product is intended for use by physicians trained and experienced in the placement of feeding tubes. Standard techniques for placement of enteral feeding tubes should be employed.
Contraindications
Use of the McLean-Ring Enteral Feeding Tube Set is contraindicated in patients with the following conditions:
- Esophageal varices
- Gastric varices
- INR (international normalized ratio) > 1.3 (at time of insertion and/or expected at time of removal)
- Anticoagulated patients (anticoagulated at time of insertion and/or expected to be anticoagulated at time of removal)
- Pathologic coagulopathies
- History of bleeding disorder(s)
- Small or large bowel obstruction(s)
- Ischemic bowel
- Peritonitis
- Esophageal stricture or obstruction
- Complete gastric obstruction
- Recent nasal, oral, esophageal, or gastric surgery or trauma
- Deviated septum
- Inability to pass the feeding tube through the nares
- Uncooperative patient
Precautions
When removing the McLean-Ring Enteral Feeding Tube through the nose or mouth, proceed very slowly.
Potential Adverse Events
Potential adverse events associated with placement and use of a McLean-Ring Enteral Feeding Tube include, but are not limited to:
- Bleeding
- Clogged or leaking feeding tube
- Sinusitis
- Premature displacement of the tube
- Aspiration
- Nasal irritation
- Sore throat
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
Instructions for Use
Adequate Peristalsis and No Gastric Outlet Obstruction
- If peristalsis is adequate and there is no gastric outlet obstruction, pass the McLean-Ring feeding tube through the external naris in the usual fashion, utilizing a water-soluble surgical lubricant.
- Advance the feeding tube until there is adequate length within the stomach to allow the feeding tube to pass through the pylorus and duodenum to the level of the ligament of Treitz.
Inadequate Peristalsis or Partial Gastric Outlet Obstruction
- If gastric outlet obstruction or inadequate peristalsis prevents the feeding tube from advancing on its own, place it over a wire guide.
- Using either a steerable catheter or a torque control catheter, under fluoroscopy advance the .038 inch (0.97 mm) diameter wire guide through the pylorus and duodenum. The wire guide enclosed with the feeding tube should be used, since this allows adequate length for effective exchange of the feeding tube.
- Advance the wire guide to well beyond the level of the ligament of Treitz.
- Leaving wire guide in place, remove catheter.
- Pre-lubricate the feeding tube prior to introduction.
- Advance the feeding tube over the wire guide by holding the end of the wire guide steady and sliding the feeding tube along the wire guide as far as it will pass. If resistance is encountered, advance the feeding tube and wire guide together as a unit. Once some progress has been made, withdraw a small amount of wire guide while maintaining slight tension on the end of the feeding tube. This allows the feeding tube to advance slowly through the lumen of the bowel, following the course of the wire guide. With this technique, the ligament of Treitz can be reached in almost all patients.
Pre-existing Gastrostomy or Jejunostomy
- If the feeding tube is to be placed through a pre-existent gastrostomy or jejunostomy tube, assess the internal diameter of the indwelling tube to assure that the tip of the feeding tube will pass through it.
- Under fluoroscopy, pass a wire guide through the gastrostomy or jejunostomy tube or stoma to the desired level in the bowel. Advance the feeding tube as described above.
NOTE: It is advisable to vigorously flush the feeding tube with water following the infusion of any of the enteral solutions. Some of these solutions may harden within the feeding tube lumen, causing concretions which may make subsequent infusions difficult.
How Supplied
Supplied sterilized by ethylene oxide gas in peel-open packages. Intended for one-time use. Sterile if package is unopened or undamaged. Do not use the product if there is doubt as to whether the product is sterile. Store in a dark, dry, cool place. Avoid extended exposure to light. Upon removal from package, inspect the product to ensure no damage has occurred.
CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician (or properly licensed practitioner).

