-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Cook Medical G06373 - SET, DIALYSIS, ACUTE PERTNL, C-PDS-851U-PT, EACH
Acute Peritoneal Dialysis Catheter Set
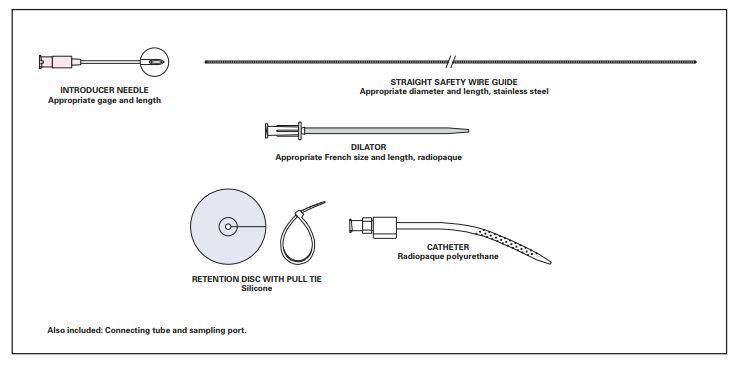
The Acute Peritoneal Dialysis Catheter is a TFE or polyurethane catheter, with a wire guide, introducer needle, and silicone retention disc.
| Order Number | Reference Part Number | Catheter Fr | Catheter Length (cm) | Catheter Sideports | Wire Guide Diameter (inch) | Wire Guide Length (cm) |
| G06373 | C-pds-851u-p | 8.5 | 8 | 44 | .035 | 30 |
Components
- 8.5 Fr Paediatric Peritoneal Dialysis Catheter
- 0.035 inch Straight Tip Wire Guide
- 9.0 Fr Dilator
- 18 gage Percutaneous Entry Needle
- Molnar Retention Disc
- 14.0 Fr Connecting Tube
Intended Use
The Acute Peritoneal Dialysis Catheter is intended for acute access to the peritoneal cavity for administration of dialysis solution.
Precautions
- This product is intended for use by physicians trained and experienced in diagnostic, interventional, and surgical techniques. Standard techniques for placement of percutaneous catheters should be employed.
- For catheters with a cuff, in order to obtain the tightest possible seal of tissues around the catheter and minimize the risk of dialysate leakage to the outside during the early phase of catheter use before tissue ingrowth into the cuff is complete, advance the assembly into the peritoneal cavity without cutting a larger hole for the wire guide.
- The potential effects of phthalates on pregnant/nursing women or children have not been fully characterized and there may be concern for reproductive and developmental effects.
Instructions for Use
- Examine the abdomen and select a site for catheter insertion that will avoid the urinary bladder, abdominal wall vessels, abdominal organs, distended bowel loops, masses or aneurysms. Catheterize the bladder to ensure that it is empty. The usual puncture site is below the umbilicus, one-third of the distance from the umbilicus to the symphysis pubis.
- Perform an antiseptic scrub of the skin and drape the puncture site area in a routine fashion.
- Infiltrate sufficient local anesthesia at the intended puncture site and then make a 4-5 mm skin incision with a #11 blade.
- Insert the introducer needle through the skin incision and advance it slowly into the peritoneal cavity.
- Insert the flexible end of the wire guide into the hub of the needle. Advance the guide 10-15 cm into the peritoneal cavity.
- Remove the needle, leaving the wire guide in place.
- Position the peritoneal dialysis catheter through the silicone retention disc. The flat side of the disc should be toward the patient.
- Introduce the peritoneal catheter over the wire guide and advance the catheter tip to the puncture site. Hold the catheter near its tip and with a firm twisting motion, advance it through the abdominal wall into the peritoneal cavity. If necessary, enlarge the skin incision with the scalpel and/or predilate the tract with the supplied dilator.
- Advance the catheter into the peritoneal cavity as far as it will go without undue force.
- Remove the wire guide.
- Attach connecting tube assembly to the peritoneal catheter.
- Attach the dialysate delivery tubing to the connecting tube.
- Secure the retention disc to the catheter at skin level by tightening the pull tie positioned on the neck of the retention disc.
- Secure retention disc to abdominal wall with a suture or tape.
- Treat the area with antiseptic ointment.
How Supplied
Supplied sterilized by ethylene oxide gas in peel-open packages. Intended for one-time use. Sterile if package is unopened or undamaged. Do not use the product if there is doubt as to whether the product is sterile. Store in a dark, dry, cool place. Avoid extended exposure to light. Upon removal from package, inspect the product to ensure no damage has occurred.
CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician (or properly licensed practitioner).


