-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

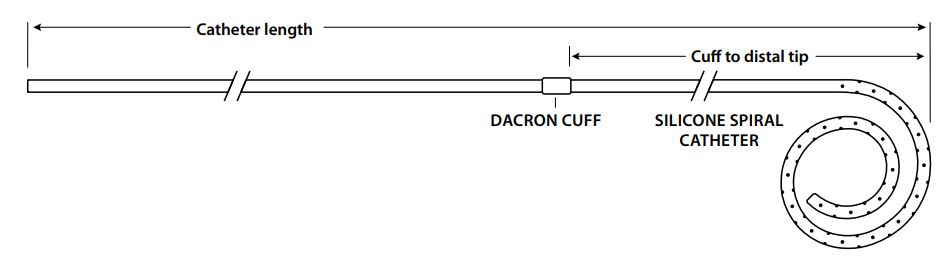
Cook Medical G08983 - CATHETER, TENCKHFF, C-TAP-9.5-31-20-SPRL-7, EACH
Tenckhoff Spiral Acute Peritoneal Dialysis Catheter
Tenckhoff Acute Peritoneal Dialysis Catheters are sideported silicone catheters with a single retention cuff, available in a range of lengths and French sizes and in either a straight or spiral tip configuration. Sets also contain an appropriately sized access needle, a wire guide and accessories for use in catheter placement.
- Peel-Away Sheath allows the catheter to be introduced percutaneously.
- The fiber cuffs are affixed to the catheter to allow tissue ingrowth.
- The sideports are spiraled.
| Order Number | Reference Part Number | Catheter (Fr) | Catheter Length (cm) | Catheter Sideports |
| G08983 | C-TAP-9.5-31-20-SPRL-7 | 9.5 | 31 | 20 |
Device Characteristics
| What MRI safety information does the labeling contain? | MR Conditional |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
Intended Use
Tenckhoff Acute Peritoneal Dialysis Catheters are intended for acute access to the peritoneal cavity.
MRI Information
Nonclinical testing has demonstrated that the Tenckhoff Acute Peritoneal Dialysis Catheter is MR Conditional. It can be safely scanned after placement under the following conditions:
- Static magnetic field of 3.0 Tesla or less
- Maximum spatial magnetic gradient field of 1600 Gauss/cm or less
- Normal operating mode: Maximum whole-body-averaged specific absorption rate (SAR) of 2.0 W/kg for 15 minutes of scanning or less (i.e., per scanning sequence)
Static Magnetic Field
The static magnetic field for comparison to the above limits is the static magnetic field that is pertinent to the patient (i.e., outside of scanner covering, accessible to a patient or individual).
MRI-Related Heating
In nonclinical testing, the Tenckhoff Acute Peritoneal Dialysis Catheter produced a maximum temperature rise of 1.7 C during 15 minutes of MR imaging (i.e., for one scanning sequence) performed in a MR 3.0 Tesla System (Siemens Magnetom Trio, A Tim System, Software Numaris/4) at an MR system reported whole-body-averaged SAR of 2.1 W/kg (associated with a calorimetry measured whole-body-averaged value of 1.7 W/kg).
Image Artifact
The image artifact does not extend from the device, but the device lumen is obscured when scanned in nonclinical testing using: GRE sequence in a 3.0 Tesla Siemens Magnetom Trio, A Tim System (Software Numaris/4) MR system with the body coil.
Precautions
- The product is intended for use by physicians trained and experienced in diagnostic, interventional and surgical techniques. Standard techniques for placement of percutaneous catheters should be employed.
- This catheter is not intended for use in patients who are not suitable for peritoneal dialysis.
Potential Adverse Events
- Bleeding
- Catheter migration
- Catheter obstruction
- Cuff erosion
- Cuff separation
- Dialysate leakage
- Dislodgement
- Fluid flow pain
- Fracture
- Hernia
- Ileus
- Infection (exit site and tunnel)
- Intestinal perforation
- Malposition
- Omental wrapping around catheter
- Peritonitis
- Sepsis
- Subcutaneous cuff extrusion
Instructions for Use
- Select a placement site that avoids both the inferior epigastric artery and any abdominal scars, so as to minimize the risk of entering the bowel adherent to the anterior abdominal wall. Both midline and paramedian abdominal sites below the umbilicus and low lateral sites have been used successfully.
- After prepping the skin as for any sterile surgical procedure, infiltrate local anesthetic.
- Make a 1 cm skin incision, followed by minimal blunt dissection down through the subcutaneous tissue.
- Fill a 20 ml syringe with sterile saline and attach it to the access needle. Advance the needle through the incision until resistance is encountered.
NOTE: At this point, the needle tip should be less than 1 cm from entering the peritoneal cavity. - Instruct the patient to tense his or her abdominal muscles, then advance the needle tip into the peritoneal cavity while simultaneously depressing the plunger on the syringe.
NOTE: Depressing the plunger on the syringe while advancing the needle should result in a jet of saline forcefully exiting the needle tip; this will make damage to any intraperitoneal soft tissues less likely. - Leaving the needle in place, remove the syringe and advance the wire guide through the needle and into the peritoneal cavity.
- Remove the needle.
- Introduce the Peel-Away sheath-introducer assembly over the wire guide well into the peritoneal cavity.
- Remove the wire guide and introducer, leaving the Peel-Away sheath in place.
- Fill or at least flush the peritoneal dialysis catheter with a heparin solution. This will reduce the likelihood of fibrin formation in the catheter during the insertion procedure.
- Insert the catheter through the sheath and into the peritoneal cavity, then peel away the sheath. Position the catheter cuff just above the linea alba (for a median insertion) or just above the rectus abdominus muscle (for a paramedian insertion).
NOTE: Some blunt dissection may be necessary to create a space for the cuff. - If necessary, one or two resorbable subcutaneous sutures may be placed before closing the initial surgical skin incision.
- Test the catheter for inflow and outflow dynamics.
How Supplied
Supplied sterilized by ethylene oxide gas in peel-open packages. Intended for one-time use. Sterile if package is unopened or undamaged. Do not use the product if there is doubt as to whether the product is sterile. Store in a dark, dry, cool place. Avoid extended exposure to light. Upon removal from packages, inspect the product to ensure no damage has occurred.
CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician (or a properly licensed practitioner).

