By Category
-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
By Brand
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Part Number
Cook Medical G14423
SKU Number
CIA2246946
Sell Unit
EACH
Ships Within
Special Order
List Price
$491.99
Product Description
Cook Medical G14423 - SET, STENT, BALLOON CATH, TAMPONADE, 086514, EACH
Kaye Nephrostomy Tamponade Balloon Set
Used for nephrostomy drainage, ureteral drainage, and dynamic tamponade to prevent hemorrhage following nephrolithotomy and percutaneous stone removal from tracts 18.0 French or larger. Intended for one-time use.
| Order Number | Reference Part Number | Catheter Fr | Catheter Length (cm) | Inflated Balloon Diameter (mm) |
| G14423 | 086514 | 14.0 | 25 | 15.5 |
Set Contains
- Flexible Stylet - Radiolucent
- Balloon Catheter - Radiopaque polyethylene
- Stent - 5.0 Fr radiopaque polyurethane 75 cm long
- Y-Connecting Tube - 14.0 Fr polyvinylchloride 30 cm long
- 10 mL syringe
CAUTION: Sterile if the package is unopened or undamaged. Do not use if package is broken.
Suggested Instructions for Using Kaye Nephrostomy Tamponade Stent
- When performing the percutaneous nephrostomy, remove the Tuohy-Borst adapter and the stent-tocatheter adapter or side-arm adapter, and advance the stent over the safety wire and into the ureter. The stent now serves as a safety wire catheter and should be secured to the skin.
- Tract dilation and placement of the nephroscopy sheath is performed in the usual manner, followed by stone manipulation.
- Upon termination of the procedure, remove the nephroscopy sheath, and the working wire if still in place.
Suggested Instructions for Using Kaye Nephrostomy Tamponade Balloon Catheter and Stent Set
CAUTION: Not recommended for dilation of a nephrostomy tract. NOTE: Remove all instrumentation related to previous procedure, with the exception of the safety wire and/or safety wire catheter. If the working wire was removed during the procedure, replace it under endoscopic vision with a wire guide. NOTE: Product will accept up to an .038 inch diameter wire guide.
- Withdraw protective sheath from balloon of catheter. Discard sheath.
- Using syringe, inject 3-4 ml of dilute contrast medium into the balloon.
WARNING: Always inflate balloon with sterile liquid. Never inflate with air, carbon dioxide or any other gas.
CAUTION: Refer to product label for maximum inflation pressure. Do not exceed the maximum inflation pressure for this balloon device. - Pass catheter over previously placed .038 inch (0.97 mm) diameter wire guide into the renal pelvis.
- Under fluoroscopic control, position the catheter so that 2-3 cm of the balloon is within the collecting system.
CAUTION: When positioning the balloon catheter, it is useful to have the collecting system opacified with contrast medium (using the ureteral catheter from the primary procedure). It is inadvisable to have the balloon catheter too far into the pelvis or down the ureter since the balloon may occlude infundibulae, or the catheter tip may irritate the pelvis (Figure A).
- To effect dynamic tamponade, inflate the balloon with 10-12 ml of dilute contrast medium. Only moderate pressure is required for tamponade.
- Replace the stent-to-catheter adapter or side-arm adapter, and the Tuohy-Borst adapter, and adjust the length of the stent in the ureter as desired. Secure the locking cap on the stent-to-catheter adapter or side-arm adapter finger tight around the stent.
NOTE: Since the distal 33 cm of the Kaye stent is sideported, the stent may be advanced fully into the bladder, thereby enhancing drainage from the renal pelvis into the bladder. - Check positioning of balloon catheter and stent with fluoroscopy. If satisfied, remove the stylet and working wire.
NOTE: Leave the safety wire and/or safety wire catheter in situ so that if you wish to replace the catheter after removal, or the catheter should become dislodged, access is maintained. - Reconfirm position with an intraoperative nephrostogram.
- To dress, place 4x4s and lap pads so the tube is gently bent. Place tape (Micro-Foam is best) over another pad (Figure B).

NOTE: Do not place tape directly on balloon.
NOTE: Leave the inflation valve in an accessible position so the balloon can later be deflated without disturbing the dressing. - Lock one leg of the Y-connecting tube onto the side-arm fitting for nephrostomy drainage. Lock the other leg onto the Tuohy-Borst adapter secured on the end of the stent for stent lumen drainage (Figure C).
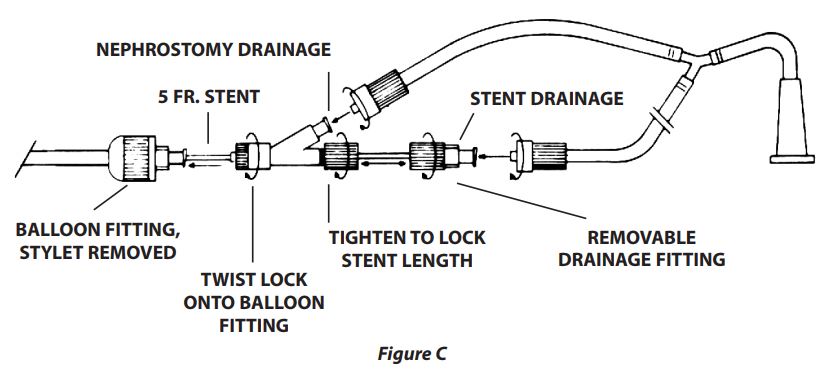
- If nephrostomy drainage is not clear at this stage and there are no pelvic lacerations, connect a normal saline irrigation fluid to the ureteral catheter and irrigate the renal pelvis, as with a TUR drip.
NOTE: As an option to the above procedure, the Kaye Nephrostomy Tamponade Stent may be placed through an existing Kaye Nephrostomy Tamponade Catheter after replacing the .038 inch diameter working wire; or the stent and catheter may be assembled prior to placement and introduced over the .038 inch diameter safety wire following the removal of the nephroscopy sheath.
Suggested Instructions for First Postoperative Day
- If urine is clear or light pink on the first postoperative day, perform a nephrostogram. If good ureteral drainage and no extravasation is evident, remove 4-5 ml of contrast medium from the balloon without disturbing the dressing.
- Observe for 4-6 hours.
Suggested Removal Instructions
- A) 1. Completely aspirate the balloon; remove entire catheter. Observe the wound site to be sure there is no bleeding.
- 2. Remove the safety wire guide and/or safety wire catheter.
NOTE: Many nephrostomy catheters can be safely removed within 36-48 hours following these suggested steps. - B) 1. Provided ureteral drainage is still desirable and tract tamponade has been effected, the Kaye catheter may be removed (see Removal Instruction A) and the Kaye stent left to drain internally. Secure the stent to the skin and clamp the proximal end.
- 2. Using a nephrostogram, check the positioning of the stent to assure that the sideports are in the renal pelvis and the distal end of the stent is in the bladder.
NOTE: The Kaye stent may also drain externally, thereby serving as a small nephrostomy tube. Drainage can be accomplished by reattaching the Tuohy-Borst adapter to the proximal end of the stent and connecting it to a connecting tube. Should bleeding occur while the stent is in place, reinsert a wire guide through the stent and reintroduce the balloon catheter over the stent. - C) 1. If the nephrostomy drainage and tract tamponade is still desirable, remove the Kaye stent and leave the Kaye catheter in place.
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |

