-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Cook Medical G19173 - SHEATH, DILATORS, FLXR, URETERAL, FUS-120020, EACH
Flexor Ureteral Access Sheath
The Flexor Ureteral Access Sheath was one of the first sheaths on the market to have a reinforced coil construction to resist kinking and compression. The sheath provides ureteral dilation and a continuous working channel for the introduction of endoscopes and instruments during ureteral access procedures. When compared to several competitor sheaths, the Flexor was shown to be less likely to buckle while advancing up the ureter.1 Use the Flexor to reduce the potential for trauma to the ureter during repeated instrument exchanges. The continuous working channel also protects delicate instruments and flexible endoscopes from damage, which can reduce the need for costly repairs.
- Ureteral access sheath
- Tapered dilator with locking hub
NOTE: Thirteen (13) cm length is intended for use in pediatric patients two (2) years of age and over.
CAUTION: Sterile if the package is unopened or undamaged. Do not use if package is broken.
CAUTION: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
| Order Number | Reference Part Number | ID Fr | Length (cm) |
| G19173 | FUS-120020 | 12.0 | 20 |
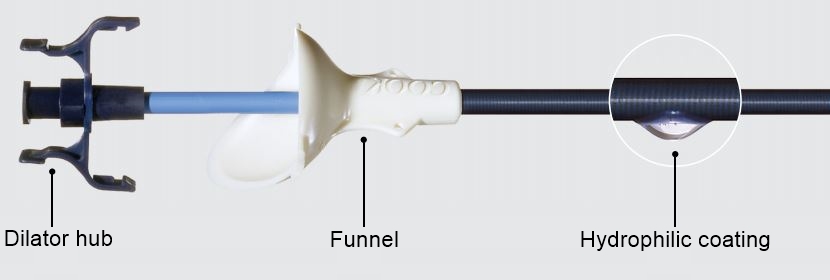
Dilator hub
The sheaths unique locking mechanism on the dilator hub secures the dilator to the sheath for simultaneous advancement of the sheath and dilator.
Funnel
The ergonomic funnel acts as a handle during insertion. The large trough facilitates instrument introduction.
Hydrophilic coating
The external surface of the sheath and the dilator tip are AQ hydrophilic coated to create a low-friction surface to ease the insertion process.
Reliability
In a randomized study, the Flexor sheath proved to be reliable for gaining ureteral access for flexible ureteroscopy.
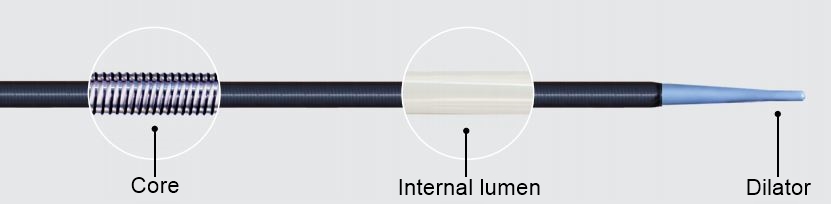
Core
The core consists of a patented coil construction to provide optimal flexibility and maximum resistance to kinking and compression.
Internal lumen
The internal lumen is PTFE lined to facilitate smooth device delivery and removal. Thin wall construction provides the largest possible internal lumen while minimizing the outer diameter.
Dilator
The dilator tapers smoothly from 6 Fr to provide gentle, one-step gradual dilation.
Suggested Instructions for Using Flexor Ureteral Access Sheath
- Place an .038 inch (0.97 mm) diameter wire guide the desired length into the ureter to establish a working tract.
- Grasp the sheath just below the instrument adapter and advance the dilator/sheath assembly over the wire guide and into the ureter.
NOTE: Be sure the dilator is securely locked onto the instrument adapter, ensuring the dilator/sheath assembly can be placed as a single unit, allowing one-hand placement. - Confirm the dilator/sheath assembly is properly placed via fluoroscopy.
- While holding the Flexor sheath in position, unlock the fitting and remove the dilator.
- Introduce the desired endoscope or instrument as needed.
NOTE: Suture may be utilized to secure the adapter externally. Suture holes are conveniently located on the instrument adapter.
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |


