-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

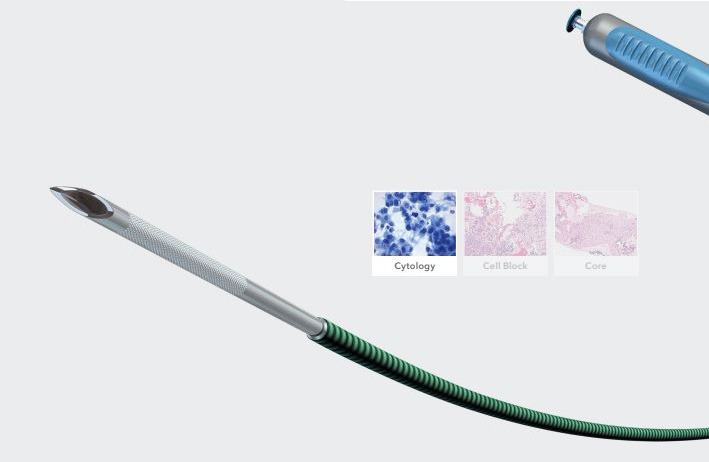
Cook Medical G31521 - NEEDLE, ULTRA SOUND, ENDOSCOPIC, ECHOTIP, EACH
EchoTip Ultra Endoscopic Ultrasound Needle
The easiest path to difficult locations.
Thanks to its highly flexible coiled sheath, the EchoTip Ultra 22 gauge gets you to difficult positions for sampling lesions. Even in torqued positions, you can acquire quality samples with the longtime best-selling EUS needle on the market.
Used to sample targeted submucosal gastrointestinal lesions through the accessory channel of an ultrasound endoscope.
| Order Number | Reference Part Number | Needle Size gage | Sheath Size Fr | Adjustable Needle Extension cm |
| G31521 | ECHO-3-22 | 22 | 5.2 | 0-8 |
Intended Use
This device is used to sample targeted submucosal gastrointestinal lesions through the accessory channel of an ultrasound endoscope.
This device is indicated for adult use only.
Notes
- Do not use this device for any purpose other than stated intended use.
- Store in a dry location, away from temperature extremes.
- Use of this device restricted to a trained healthcare professional.
- Please read all instructions before using this device.
Contraindications
- Those specific to primary endoscopic procedure to be performed in gaining access to desired site.
- Coagulopathy
Potential Complications
Those associated with gastrointestinal endoscopy include, but are not limited to: perforation, hemorrhage, aspiration, fever, infection, allergic reaction to medication, hypotension, respiratory depression or arrest, cardiac arrhythmia or arrest.
Precautions
- Refer to package label for minimum channel size required for this device.
- When targeting multiple sites, replace device for each site.
- Needle must be fully retracted into sheath and thumbscrew on safety ring must be locked at 0 cm mark to hold needle in place prior to introduction, advancement or withdrawal of device. Failure to retract needle may result in damage to endoscope.
System Preparation
- Examine syringe. It has two plunger locks that must be depressed to advance plunger. Tip of syringe has a Luer lock fitting with a stopcock on the side port. Air can be exchanged when stopcock is in "open" position, aligned with syringe.
- Prepare syringe as follows:
- With stopcock in open position, depress plunger locks and fully advance plunger into syringe.
- Turn stopcock 90 to "closed" position.
- Pull plunger back until it is locked into place at desired setting, creating a suction.
- Set prepared syringe aside until aspiration biopsy is desired.
- Advance device into ultrasound endoscope to determine preferred sheath length. To adjust length, loosen thumbscrew lock on sliding sheath adjuster and slide until preferred length is attained.
Note: Reference mark for sheath length will appear in sliding sheath adjuster window. Tighten thumbscrew on sliding sheath adjuster to maintain preferred sheath length.
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |