-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Cook Medical G44153 - ANSEL, 5FR 45CM FLEXOR SHEATH, EACH
Flexor Ansel Guiding Sheath with Check-Flo Valve
FLEXOR ANSEL - CHECK-FLO VALVE
The Flexor Ansel offers gentle curves, soft tip, and hydrophilic coating which enhance trackability while .018" and .038" compatible dilators allow choice in platform.
Flexor Introducers and Guiding Sheaths are designed to perform as a guiding sheath and/or introducer sheath. Flexor Introducers and Guiding Sheaths incorporate a Flexor shaft with a hemostasis valve and are provided with one or more dilators.
- Used to introduce balloon, closed and nontapered for catheters or other devices.
- Uniquely fabricated sheath design provides maximum flexibility without kinking or compression.
- 0.018 and 0.038 inch wire compatible dilators.
- Preformed curve combined with the distance involved allows atraumatic soft target vessel.
- Short dilator tip enhanced trackability and facilitates placement of the Congress introduced in tight spaces.
- Radiopaque band, incorporated within sheath material, determine the exact location of the sheath tip for precise remote location.
- Check-Flo hemostasis valve accepts a variety of sizes while preventing blood reflux and air aspiration.
- AQ hydrophilic coated on both shell and dilator are providing a lubricious surface, easing introduction.
- Providing sterile in peel open package. For a period of use.
| Order Number | Reference Part Number | FR | ID mm | Length cm | Accepts Wire Guide Diameter inch | Tip Configuration |
| G44153 | KCFW-5.0-18/38-45-RB-ANL0-HC | 5 | 1.9 | 45 | .018/.038 | renal double |
Intended Use
Flexor Introducers and Guiding Sheaths are intended to introduce therapeutic or diagnostic devices into the vasculature, excluding coronary and neuro vasculature.Used to introduce balloons, closed and non-tapered end catheters or other diagnostic and interventional devices.
Flexor Technology
Optimizing access throughout the body. Where access is difficult, Flexor adapts. Where delivery is demanding, Flexor rises to the challenge.
Evolution of The Guiding Sheath
The first group of physicians in the world tackling carotid stenting asked Cook to create longer, soft-tipped sheaths-instead of catheters-to enter the fragile vasculature of the carotid artery. We delivered with the development of the guiding sheath-a two-in-one device that provides direct access to the body without the need for an introducer sheath. We expanded this line beyond carotid guiding sheaths to make products for the iliofemoral and renal arteries to decrease your procedural time and lessen the possibility for complications during interventions.
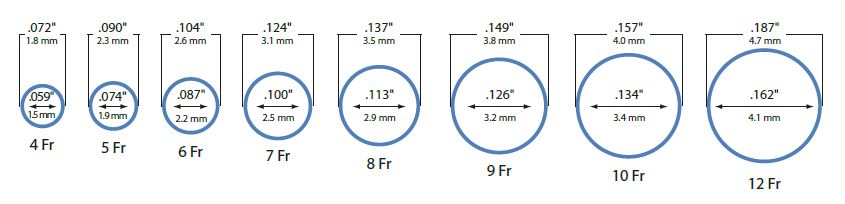
When choosing guiding sheaths, please remember that sizes are measured differently than the guiding catheter line. The labeled size of a guiding sheath refers to the inner diameter. The labeled size of a guiding catheter refers to the outer diameter. An 8.0 French guiding catheter is equivalent in diameter to a 6.0 French guiding sheath. And, unlike guiding catheters, guiding sheaths have inner dilators or obturators that taper down for insertion over the wire guide. This inner dilator is removed after the guiding sheath is inserted into the body.
Unlimited Results Never Come from Limited Resources
Durability
Patented coil-reinforced design provides optimal flexibility and trackability in places where non-reinforced sheaths would kink.Flexor technology is available in a broad product offering to ensure that you are not restricted by limitations.
Greater Visibility
Radiopaque band seamlessly incorporated in the tip of the sheath material identifies the precise location of the introducer under fluoroscopy.
Unsurpassed Selection
Large, low-friction PTFE-coated lumens range from 4.0 French through 12.0 French.
Precautions
- This product is intended for use by physicians trained and experienced in diagnostic and interventional techniques. Standard techniques for placement of vascular access sheaths should be employed.
- In order to ensure device compatibility, choose a sheath size large enough to accommodate the maximum outer diameter of any devices that will be placed through the sheath.
- All interventional or diagnostic instruments used with this product should move freely through the valve and sheath to avoid damage.
- Do not attempt to heat or reshape the device.
- When inserting, manipulating or withdrawing a device through the sheath, always maintain sheath position.
- When puncturing, suturing or incising the tissue near the sheath, use caution to avoid damaging the sheath.
- The potential effects of phthalates on children or pregnant/nursing women have not been fully characterized and there may be concern for reproductive and developmental effects.
Potential Adverse Events
Adverse events that may be associated with the use of an introducer set include, but are not limited to:
- Bleeding
- Extravasation
- Hematoma
- Vessel laceration
- Vessel perforation
- Local inflammation
- Local pain
- Access site infection
- Distal embolization
Instructions for Use
Sheath Introduction
- Ensure that the inner diameter (ID) of the sheath is appropriate for the maximum diameter of the instruments to be introduced.
- Using the side-arm of the valve, flush the sheath by filling the sheath assembly completely with heparinized saline.
- Flush the dilator with heparinized saline.
- Insert the dilator completely into the sheath. If the sheath has a Tuohy-Borst valve, tighten the valve around the dilator.
- If the device has hydrophilic coating, activate the coating by wetting the outer surface of the device with heparinized saline. NOTE: For best results, maintain wetted condition of device during placement.
- Using standard Seldinger technique, access the target vessel with the appropriate needle.
- Insert a wire guide into the vessel through the needle.
- Leave the wire guide in place and remove the needle.
- Insert the dilator/sheath assembly over the wire guide.
- Remove the wire guide and dilator, then aspirate and flush through the sheath side-arm.
- Insert appropriately sized device(s) as needed.
Sheath Removal
- Insert a wire guide until its tip extends at least 10 cm past the tip of the sheath.
- Remove the sheath. Avoid applying traction to hub during removal. If resistance is anticipated or encountered during withdrawal of the Flexor sheath, consider reinserting the dilator and removing the sheath and dilator as a unit.
- Remove the wire guide.
How Supplied
Supplied sterilized by ethylene oxide gas in peel-open packages. Intended for one-time use. Sterile if package is unopened or undamaged. Do not use the product if there is doubt as to whether the product is sterile. Store in a dark, dry, cool place. Avoid extended exposure to light. Upon removal from package, inspect the product to ensure no damage has occurred.





