By Category
-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
By Brand
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Part Number
Cook Medical G46220
SKU Number
CIA2263736
Sell Unit
BOX
Ships Within
24 Hours
List Price
$889.96
Product Description
Cook Medical G46220 - LOOP, CUTTING, COOK, CL-2712-KS1, 5/BX
Cook Cutting Loop
Compatible with STORZ Brand Single-Stem Resectoscope
The Cook device is a sterile monopolar electrode designed to deliver radio frequency energy that is supplied by an electrosurgical generator cleared for medical use.
- Intended for one time use.
- Do not re-sterilize.
- Store in a cool, dry place.
| Order Number | Reference Part Number | Fits Fr | Wire Diameter inch | Loop Angle degrees |
| G46220 | CL-2712-KS1 | 27 | 0.012 | Straight |
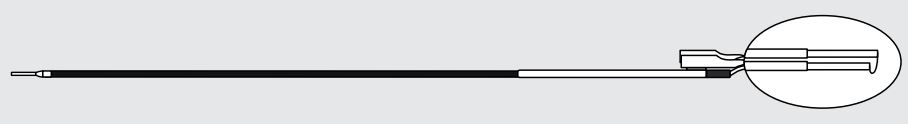
CAUTION: Sterile if the package is unopened or undamaged. Do not use if package is broken.
Indications for Use
The Cook device is indicated for the ablation/coagulation of soft tissue and is intended for use with compatible resectoscopes.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Potential Complications
Complications could include local and/or systemic infection, thermal damage to surrounding structures, local hematoma, dissection and perforation, and patient discomfort during and/or after energy application.
Warnings and Precautions
- All medical staff should carefully review product labeling and instruction sheets before using this device. Inappropriate use of the instrument could adversely affect the procedure or cause injury to the patient and/or surgeon.
- Refer to the applicable operating and maintenance manual for the resectoscope and electrosurgical generator being used.
- This device should only be used by a physician who is familiar with the use of electrosurgical instruments, devices, and power generators. Consult the medical literature regarding techniques, complications and hazards prior to any endoscopic procedure.
- Do not bend or change the angle or shape of the distal end. To do so may cause poor function, damage to the resectoscope and injury to the physician and/or the patient.
- Power settings vary greatly depending on the surgeons preferences, technique, type of electrosurgical generator used and tip style. The maximum rated voltage for this device is 3000 Vp-p (AC). As a general guideline, the following power settings can be used:
- In the "cut" mode, the power setting should be started low and increased gradually until the desired electrosurgical effect is achieved. Never exceed 280 watts.
- In the "coagulation" mode, the power setting should be started low and increased gradually until the desired electrosurgical effect is achieved. Never exceed 200 watts. Caution must be taken to prevent arcing on high coagulation settings.
- If there is little or no tissue effect, check the generator, power cables, patient ground and the device.
- Constant irrigation is required throughout the procedure. The distal tip of the device should be kept in view and submerged in the irrigant at all times.
CAUTION: Only non-conductive irrigants should be used. - During the procedure with a resectoscope, make sure that the HF current is only activated under visual control to avoid inadvertent burns.
- Immediately discontinue use if breaks or fractures appear in the device. These conditions may allow undirected emission of electrical energy, rendering the device useless and potentially causing harm to surrounding tissues.
- Do not use if there are breaks, scratches, and cracks in the insulation on the electrode. Use of the electrode with damaged insulation may cause unintended electrosurgical burns.
- Care should be taken to avoid severe impacts, side stresses or bends at sharp angles.
- When endoscopic devices are used together, ensure that any isolation or ground is not violated.
Sterilization
| Device Packaged as Sterile: | Yes |
| Requires Sterilization Prior to Use: | No |
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
Directions for Use
- Remove the device from the package and examine it for damage. Do not use if there are visible signs of damage, or if insulation is not intact.
- Assemble the working element and resectoscope according to the instructions provided by the resectoscope manufacturer.
- Insert the device into the working channel of the resectoscope in a manner consistent with the instructions of the resectoscope.
- Ensure that the electrode is securely locked into position by pulling carefully on the electrode stabilizer sleeve (if applicable). Do not squeeze the stabilizer sleeve.
- Attach resectoscope to the electrosurgical generator according to the manufacturers recommendations.
- Insert combined assembly into the resectoscope sheath and position at the point where initial application of electrical energy will be delivered.
- The electrosurgical generator should be set at power levels consistent with standard ablation/resection procedures.
CAUTION: Power setting may not exceed the limits noted in #5 of the precautions section. - The power level can be adjusted and varied slightly for different patients and also when tissue is being removed on subsequent sweeps.
- Maintain constant irrigation through the resectoscope using sterile irrigant. Keep the distal tip of the device in view and submerged in the irrigant at all times.
- If tissue adheres to the working end, cleaning can be accomplished by switching to a coagulating setting of 200 watts maximum and engaging current without tissue contact. The working end needs to be fully submerged in irrigation solution. Cleaning may also be accomplished by removing the device and wiping it with a sterile, lint-free cloth.
- After use, the electrode may be a potential biohazard. Handle and dispose of in accordance with accepted medical practice and applicable local, state and federal laws and regulations.

