-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Cook Medical G54914 - TUBE, TRACH, TAPERED, VERSATUBE, SZ 7, EACH
Versatube Tapered Tracheostomy Tube with Disposable Inner Cannula
The VersaTube Tapered Tracheostomy Tube consists of an outer cannula, inner cannula, obturator, and tracheostomy tube strap.

| Order Number | Reference Part Number | Tracheostomy Tube ID (mm) | Tracheostomy Tube OD (mm) | TracheostomyTube | Angle degrees | Cuff Resting Diameter (mm) | Tracheostomy Tube Color Code |
| G54914 | C-VT-7 | 7 | 10 | 78 | 96 | 25 | 6/Green |
Features & Benefits
NOTE: Tracheostomy Tube ID is the inside diameter of the outer cannula. OD is the outside diameter of the outer cannula (not including cuff features). Length is the distance from the back of the neck plate to the distal tip of the tube. Cuff OD is the outer diameter of the cuff at the resting diameter.
- Long length options enable use in a wider variety of patient types.
- Tapered distal tip creates a smooth transition from loading obturator to tracheostomy tube.
- Colour-coding system facilitates simple and efficient identification of desired tube and inner cannula size.
- Soft PVC blend material gently conforms to patient anatomy to maximise comfort and help prevent damage to internal structures.
Components
- Tracheostomy Tube
- Inner Cannula
- Obturator
Treatment Versatility
Long length (sizes available up to 98 mm) enables use in a wider variety of patient types, from normal to those with larger necks, while a 15 mm connector on the main tube makes inner cannula use optional.
Decreased Risk of Trauma
Tapered distal tip creates a smooth transition from loading obturator to tracheostomy tube outer diameter, minimizing insertion force and the potential for tracheal wall trauma.
Ease of Use
Familiar ISO-standard sizing creates easy conversion from ETT to tracheostomy tube, and convenient color-coding system facilitates simple and effi cient identifi cation of desired tube and inner cannula size.
Enhanced Patient Comfort
Soft PVC blend material gently conforms to patient anatomy to maximize comfort and help prevent damage to internal structures
Intended Use
The VersaTube Tapered Tracheostomy Tube is intended to provide an artificial airway to establish airway patency and to provide maintenance of the airway.
Precautions
- This product is designed to be used in Percutaneous Dilational Tracheostomy (PDT) procedures and Balloon Assisted Tracheostomy procedures. Standard PDT or Balloon Assisted Tracheostomy techniques should be employed. Sterile technique should be adhered to for the handling and insertion of the tracheostomy tube.
- The VersaTube Tracheostomy Tube is classified as a single use device. The manufacturer recommends that the tracheostomy tube usage does not exceed twenty-nine days.
- For ventilator-dependent patients, a replacement tracheostomy tube should be kept at bedside and cuff inflation should be monitored regularly.
- Insertion of this tracheostomy tube in morbidly obese patients may result in tube obstruction or false passage of the tube.
- If lubricant is used prior to tracheostomy tube insertion, ensure that lubricant does not occlude the tubes lumen; lumen occlusion may prevent adequate ventilation.
- Patients breathing gas should be adequately humidified to minimize encrustation of the tracheostomy tube and/or inner cannula lumen and prevent mucosal damage.
- The inner cannula should be checked and replaced at regular intervals to avoid blockage which may reduce or obstruct airway lumen.
- Do not reposition the in situ tracheostomy tube while the cuff is inflated; doing so may result in trauma to the stoma and/or trachea.
- During anesthesia, nitrous oxide may diffuse into the cuff, causing an initial increase, with subsequent decrease, in cuff pressure. This can result in pressure trauma to the trachea and loss of airway seal, resulting in aspiration of subglottic secretions into the lungs.
- Prior to removal of cuffed tracheostomy tubes, ensure that all air is completely removed from the cuff; failing to do so may result in pain to the patient and damage to the trachea and/or stoma.
- During and after attachment of the breathing system to the tracheostomy tube connector, avoid excessive rotational or linear forces on the tube; application of such forces may result in tube kinking, disconnection, or occlusion
Instructions for Use
Tube Preparation
- Select the appropriately sized tracheostomy tube.
- Test the cuff and infl ation system for leakage.
- With the cuff infl ated, taper the cuff back by gently moving away from the distal tip of the outer cannula toward the swivel neck plate as the residual air is removed by defl ation. This will ease insertion and guard against cuff perforation by sharp cartilage edges.
NOTE: Do not use sharp instruments such as forceps or hemostats to taper the cuff; doing so may result in cuff damage. - Ensure that the cuff is fully defl ated.

- Remove inner cannula. (Picture 1)
Insertion
- With the inner cannula removed from the tube, insert an appropriately sized loading dilator into the tracheostomy tube. The tapered section of the loading dilator should extend from the distal tip of the tracheostomy tube by approximately 2 cm.
NOTE: Water-soluble lubricant can be applied to the outer cannula, cuff and protruding portion of loading dilator to facilitate insertion. - Perform the dilational tracheostomy procedure per the introducer system manufacturers instructions
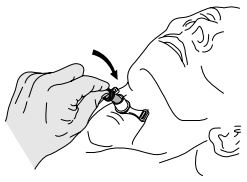
- Using aseptic technique, insert the inner cannula into position. (Picture 2)
NOTE: Two sterile inner cannulas are provided. The outer cannula can be used independently (without the inner cannula in place). - Infl ate the low-pressure cuff by injecting air into the Luer valve of the infl ation line using a syringe. Selection of a cuff infl ation and defl ation method is at the discretion of the physician/hospital.
- Switch the breathing apparatus from the endotracheal tube to the tracheostomy tube and verify adequate ventilation.
- Secure the tracheostomy tube per hospital protocol.
NOTE: A tracheostomy tube strap is included with this device.
CAUTION: This product is composed of soft materials to conform to tracheal tissue for performance and patient comfort. Simple precautions in handling this tube during insertion and while in place will facilitate proper function and minimize tears and breaks in the infl ation system. Avoid pulling or
manipulating the infl ation line, as it is designed to conduct and hold air as part of the cuff infl ation system. It is recommended that the infl ation line be maintained in a position allowing for patient mobility without placing tension on the line-to-cannula junction.
Cuff Deflation and Tube Removal
- Before removing the tube, the cuff should be completely defl ated. This will ensure that the cuff passes through the stoma with minimal resistance.
NOTE: Accumulated secretions above the cuff may need to be suctioned before defl ating the cuff (with a syringe), unless suctioning is contraindicated. - To defl ate the cuff, withdraw air slowly from the Luer valve of the infl ation line using a syringe. After the cuff is fully defl ated, slowly remove the tracheostomy tube.
How Supplied
Supplied sterilized by ethylene oxide gas in peel-open packages. Intended for one-time use. Sterile if package is unopened or undamaged. Do not use the product if there is doubt as to whether the product is sterile. Store in a dark, dry, cool place. Avoid extended exposure to light. Upon removal from package, inspect the product to ensure no damage has occurred.
CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician (or properly licensed practitioner).

