-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Covidien CP435 - Suture Surgipro Blue GS-24 1 30" 36/BX

Covidien CP435 SURGIPRO Monofilament Polypropylene Suture, Taper Point, Size 1, 30", Needle GS-24, 1/2 Circle, Blue (Pack of 36)
Uncoated Surgipro II sutures (clear or pigmented) are inert, non-absorbable, sterile sutures composed of an isotactic, crystalline stereoisomer of polypropylene and contain polyethylene. Surgipro II sutures are indicated for use in general soft tissue approximation and/or ligation, including use in cardiovascular, ophthalmic and neurological surgery. The suture is pigmented blue to enhance visibility.
Surgipro sutures are uncoated, inert, non-absorbable, sterile sutures composed of an isotactic, crystalline stereoisomer of polypropylene and contain polyethylene. These sutures are available dyed blue for visibility or undyed clear. Surgipro sutures are indicated for use in general soft tissue approximation and/or ligation, including use in cardiovascular, ophthalmic, and neurological surgery.
The advanced extrusion process of the polypropylene molecule gives the suture:
- Uniform diameter
- Maximum flexibility of the strand
- Excellent security with snug and flattened knots
- Minimal memory and reduced "pig-tailing"
- Consistent knotting strength
Surgipro II Suture
Polypropylene Monofilament Suture Used for Cardiovascular and Peripheral Vascular Procedures
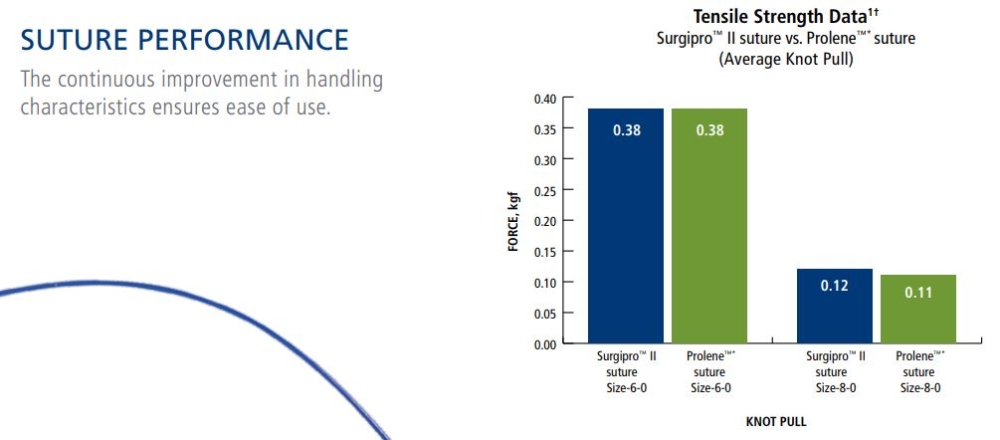
Surgipro II suture is a brand you have trusted for many years with several product innovations. Our history of innovation and experience provides you with consistent and reliable outcomes. With us, your patients life can be safe and secure.
Covidien's Full Line of Product Offerings Can Meet All Your Surgical Needs
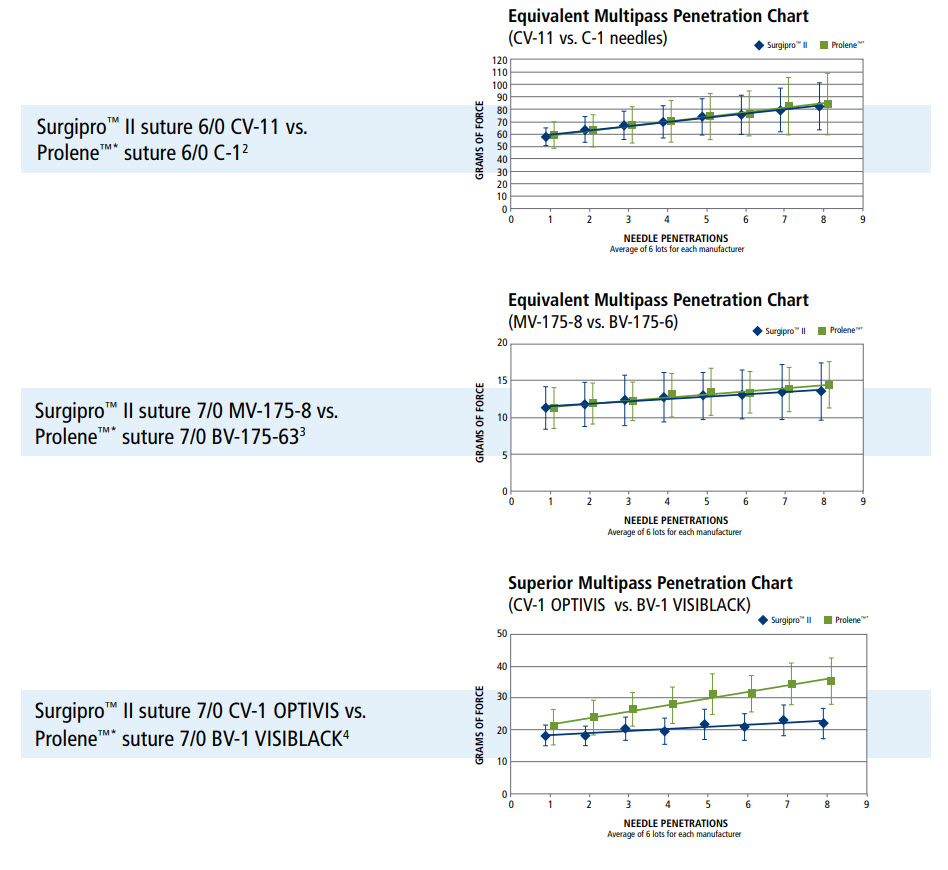
Needle Performance
- Surgalloy premium needle alloy designed to enhance needle strength
- NuCoat coating maintains needle sharpness over the duration of the procedure, resulting in consistent penetration, pass after pass
- Optivis surfaced darkened needles provide maximum visibility without affecting needle performance
- Flat-pressed needles: CVF and KVF needles are available for enhanced stability in the needle driver
- Taper-cutting needles (KV) have a taper body with a cutting tip
- Cardiopoint needles:
- Specialized cardiac needles less likely to break when operating at difficult angles
- Y needles with smaller cutting tip
Indications:
SURGIPRO II - SURGIPRO polypropylene sutures are indicated for use in general soft tissue approximation and/or ligation, including use in cardiovascular, ophthalmic and neurological surgery.
Actions:
SURGIPRO II - SURGIPRO polypropylene sutures elicit a minimal acute inflammatory reaction in tissues, which is followed by gradual encapsulation of the suture by fibrous connective tissue.
Tensile Strength:
SURGIPRO II - SURGIPRO polypropylene sutures are non absorbable and no significant change in strength retention is known to occur in vivo.
Multipass Penetration Comparisons
Packaging:
- SURGIPRO II polypropylene sutures are available either undyed (clear) or dyed with Copper Phthalocyanine Blue, in sizes 8-0 (0.4 Metric) through 3-0 (2 Metric).
- SURGIPRO polypropylene sutures are available either undyed (clear) or dyed with Copper Phthalocyanine Blue, in sizes 10-0 (0.2 Metric) and 8-0 (0.4 Metric) through 2 (5 Metric). The sutures are supplied sterile, in pre-cut lengths and ligating reels, non needled or affixed to needles using both permanent and removable needle attachment techniques: in one, two, and three dozen box quantities.
- SURGIPRO II polypropylene sutures are also available in presentations containing PTFE (polytetrafluorethylene) pledgets for use as a pad between the suture and the tissue surface to increase the load bearing area.
- PTFE (polytetrafluorethylene) pledgets for use as a pad between the suture and the tissue surface to increase the load bearing area.
- Bead & collar components to anchor the ends of the suture for subcuticular closure or for use as tendon sutures.
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |

Covidien #CP455, SUTURE, SURGIPRO, BLUE, 1, 30", GS-25, 36/BX
$376.96 per BOX

Covidien #CP415, SUTURE, SURGIPRO, BLUE, 1, 36", HGS-22, 36/BX
$531.60 per BOX

Covidien #8886446371, SUTURE, NOVAFIL, BLUE, 1, 30", T-25, 36/BX
$472.72 per BOX

Covidien #CP535, SUTURE, SURGIPRO, BLUE, 1, 40", GS-24, 36/BX
$276.16 per BOX

