-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Covidien GIA10048S - GIA 100 - 4.8MM Single Use Reloadable Stapler, 3/BX
Directional Stapling Technology provides improved staple formation and enhanced security in tissue closure as shown by the study published in January 2005 on the DST Series TA instruments. The purpose of the second research study discussed in this white paper was to document in an in vivo study that the Directional Stapling Technology produces similar results with the DST Series GIA stapler.
Experimental Design
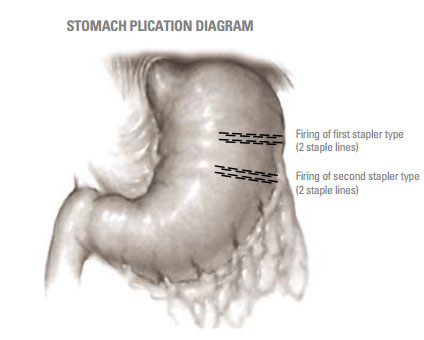
Sixteen, non-surviving canines (17-27kg) of bothsexes were used to evaluate the performance ofeach of the two types of staplers. Each type ofstapler was fired in parallel 50mm apart along thegreater curvature of the canine stomach, creatingtest segments. The order of firing was randomizedto minimize anatomical bias. Each test segment wasexcised, and a special clamp applied across theopen end of the test segment, and down the center,isolating each staple line for testing. Each staple linewas independently quantitated for resistance tofluid leakage, staple geometry and staple twist.All results were analyzed for statistically significant(p = 0.05) differences.
Resistance to Leakage
The thickness of stomach tissue was measured with a spring-loaded caliper. Each stomach received a minimum of one firing (two staple lines) from each type of stapler across both walls of the stomach (Figure 1). All aplications were transverse and 70mm in length. After firing the first staple line, the other type of stapler was used to plicate the stomach again 50mm lateral to the first plication. This created test pockets of stomach tissue sealed on the lateral edges by the staple lines. These test pockets were excised from the stomach and used to quantify the pressure required to cause water leakage. The open end and center of the test pocket was clamped and an infusion pump with an in-line pressure monitor was used to independently fill each half of the test pocket through an 18 gauge needle. Thus each staple line was tested independently. Colored water was infused at a rate that resulted in a 25mm Hg increase in pressure every 30 seconds. Failure was recorded as the pressure which resulted in steady leakage.


Features and Benefits
1.Tear Drop Anvil/Strongest Beam Available
- Reduces deflection, eliminates pin, improves staple formation.
2.Lock Lever on Cartridge Side
- Improved access.
3.Ergonomic Inlay Grips
- Better control.
4.Rear Hinge
- One-handed positioning.
5.Adjustable Firing Knob
- Versatility - may be fired from either side.
6.Pinless for atraumatic placement
- Avoids trauma associated with placement pin.
7.Directional Staple Technology
- Improved staple formation and staple line security, especially in challenging applications.
FAQs
1.Will Directional Staple Technology be incorporated into all Staplers?
Directional Staple Technology has proven to improve the performance, reliability and manufactureability of TA and GIA Series Staplers. The effectiveness of Directional Staple Technology in other staples will be assessed as these products are developed.
2.Why reinvent your current line of staplers? I have no problems with the staplers I use now?
We continually strive to enhance and develop our existing product lines to provide the highest quality, cost effective, consistent, and reliable technologies, all focused on improving outcomes. Previous linear staplers and GIA Staplers are models that were designed and released in 1992imagine if cars or computers and the internet were still functioning at 1992 standards.
3.In your literature, you reference less-than-desirable staple formation?
During our research, surgeons suggested that, in certain applications, existing technologies had limitation, as demonstrated by the illustration. This may be encountered in thick tissue, where there is cartridge to anvil deflection or misalignment (demonstrate this point using the DST Series sell sheet). In the automotive industry, ABS Technology evolved to enhance performance in challenging braking situations. Similarly, DST Series staplers evolved to enhance performance in challenging tissue applications. We continually strive to enhance and develop our existing product lines to provide the highest quality, cost effective, consistent, and reliable technologies, all focused on improving outcomes.
4.Does Directional Staple Technology cause necrosis?
30 years of clinical data support a "B" shaped staple. The Directional Staple Technology still delivers a B shaped staple, just does so more consistently and reliably, particularly in thicker tissue applications.


