-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

C.R. Bard 0141975 - PowerLoc MAX Power-Injectable Infusion Set without Y-injection Site, 19 Gauge x .75", 25/CS
The PowerLoc Safety Infusion Set Family is designed with both the clinician and the patient in mind. The product is indicated for the administration of fluids, drugs and blood sampling via a surgically implanted vascular port. The PowerLoc Safety Infusion Set Family is the ideal device for accessing PowerPort devices. This power-combo enables contrast agents to be power-injected. As a result, tissues show up more clearly in CECT scans, making it easier to monitor patient condition.
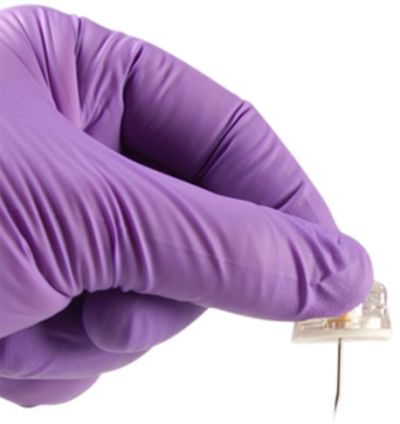
Safety Mechanism
The safety mechanism is housed in the plastic base. Once the needle has been pulled through the base the mechanism covers the needle point and locks it into the plastic base. The safety mechanism protects clinicians and patients from accidental needlestick injuries during needle removal, transport and disposal of the PowerLoc Max.

Features and Benefits
- Stable needle base for patient comfort during dressing.
- Dual lumen port compatible to enable simultaneous infusions through dual lumen ports.
- Audible "click" and visual indication of lockout to ensure safety device has been activated.
- Safety feature covers needlepoint to reduce risk of injury and exposure to bloodborne pathogens.
- Smooth and simple, single-step safety activation.
- Safety feature conforms to current user technique.
- Wingless design for site care.
Designed for Patient Comfort
- Smallest footprint of all Bard Safety Infusion Sets.
- Non-absorbent patient comfort pad.
- Better dressing profile.
- Lower insertion and penetration force than other non-siliconized port access needles.
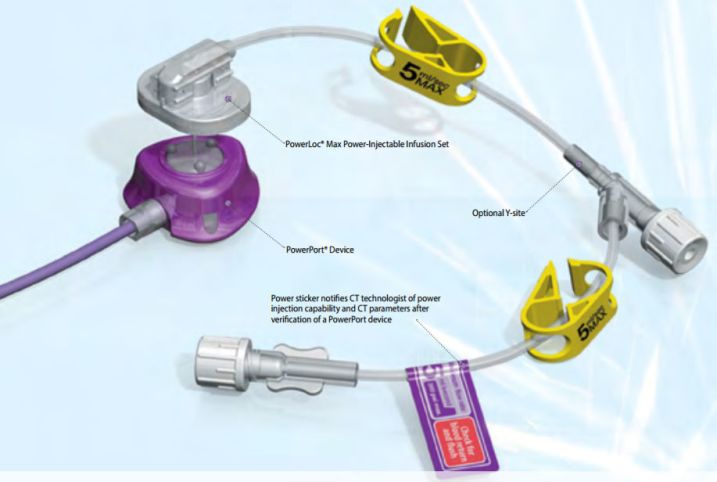
Indications For Use
The PowerLoc Safety Infusion Set Family of devices is intended for use in the administration of fluids and drugs, as well as blood sampling through surgically implanted vascular ports. The PowerLoc Safety Infusion Set Family of devices are also indicated for power injection of contrast media into the central venous system only with an implanted port that is also indicated for power injection. The maximum recommended infusion rate at 11.8 cPs is 5 mL/second for 19 G needles, 5 mL/second for 20 G needles, and 2 mL/second for 22 G needles.
Contraindications
- DO NOT USE, if the presence of a device related infection, bacteria, or septicemia is known or suspected.
- DO NOT USE, if local tissue factors will prevent proper device stabilization and/or access.
Insertion
- Prepare the port site for sterile accessing following institution protocol.
- Prime infusion set using aseptic technique following institution protocol.
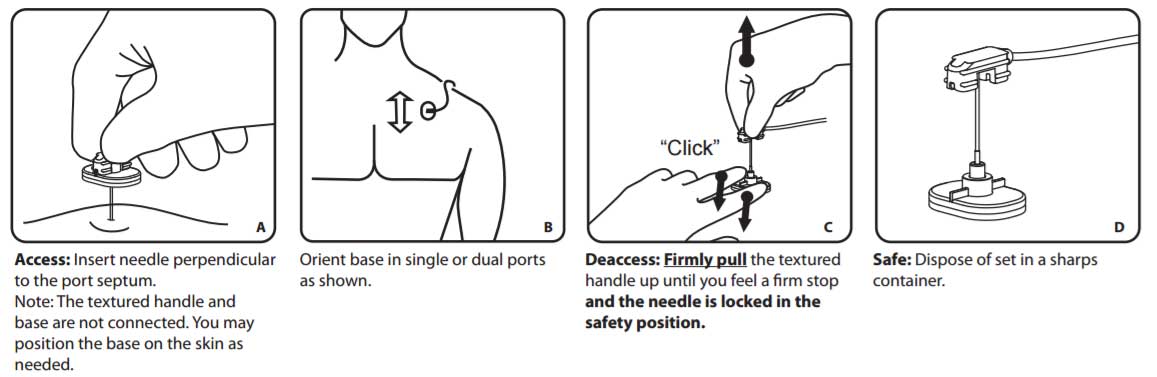
Insertion method
- Verify correct needle placement by aspirating for blood.
- Flush per institution protocol and close clamp on extension tubing.
- Secure needle to site following institution protocol. Do not manipulate the needle once it is in the septum.
Warnings!
- Fully tighten all connections, Y-site end caps, or needleless connectors before use. Failure to attach an end cap or appropriate needleless device after removing a male Luer locking end cap or needleless connector can result in an embolism or bleeding.
- Intended for Single Use. DO NOT REUSE. Reuse and/or repackaging may create a risk of patient or user infection, compromise the structural integrity and/or essential material and design characteristics of the device, which may lead to device failure, and/or lead to injury, illness or death of the patient.
- Failure to use the safety mechanism of the device correctly, when removing needle from port site could result in needle tip re-emerging from the base, resulting in an accidental needlestick with a contaminated needle. A needlestick with a contaminated needle may cause infectious disease.
- Verify needle length is correct based on port reservoir depth, tissue thickness and the thickness of any dressing beneath the bend of the needle; if too long, needle and/or port may be damaged at insertion; if too short, needle may not completely pierce port septum, and medication may be delivered into surrounding tissue and/or needle may be blocked.
- Do not alter the device.
- After use, this product may be a potential biohazard. Handle and dispose of in accordance with accepted medical practice and applicable local, state and federal laws and regulations.

C.R. Bard #0142075, PowerLoc MAX Power-Injectable Infusion Set without Y-injection Site, 20 Gauge x .75", 25/CS
$236.71 per CASE

C.R. Bard #0652034, PowerLoc Safety Infusion Set without Y-injection Site, 20 Gauge x .75", 20/CS
$199.64 per CASE

C.R. Bard #0142015, PowerLoc MAX Power-Injectable Infusion Set without Y-injection Site, 20 Gauge x 1.5", 25/CS
$233.55 per BOX

C.R. Bard #0142210, PowerLoc MAX Power-Injectable Infusion Set without Y-injection Site, 22 Gauge x 1", 25/CS
$233.55 per BOX

