-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Smith & Nephew 66800559 - Durafiber Protease dressing 5cm x 5cm
DURAFIBER Soft Sterile Non-Woven Pad or Ribbon Dressings
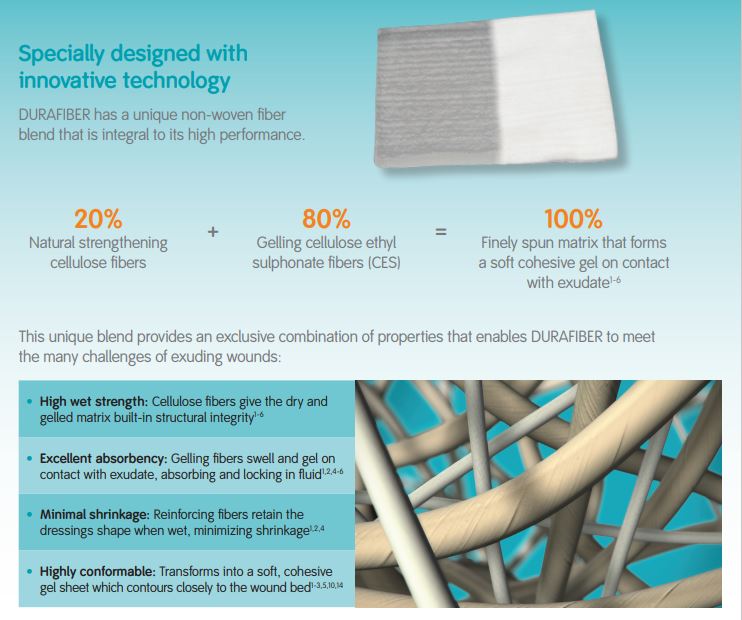
DURAFIBER is a soft sterile non-woven pad or ribbon dressing composed of cellulose ethyl sulfonate fibres.
This highly absorbent and conformable dressing is designed to rapidly form a clear, cool gel on contact with wound fluid. This gel absorbs excess fluid, locks exudate away from the wound, provides a moist environment to support autolytic debridement and conforms intimately to the wound bed.
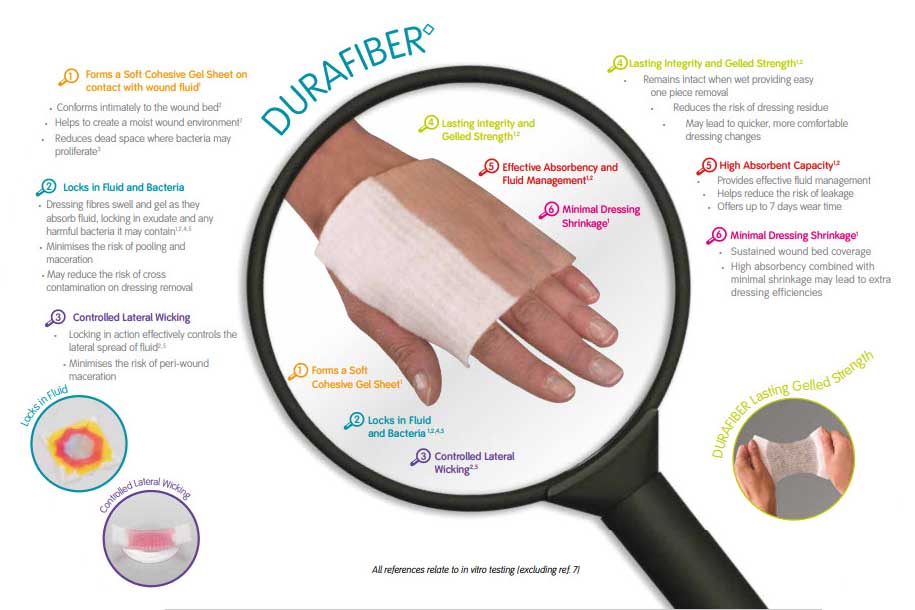
The high integral wet strength of DURAFIBER facilitates easy one-piece removal from moist wound beds and cavity wounds; minimising trauma to the wound and pain to the patient on removal. DURAFIBER can be used in conjunction with ALLEVYN, PROFORE or PROGUIDE.
Strength in Design
Life with a wound
Living with a chronic wound can be an uncomfortable and distressing experience. Dressings designed to protect and promote healing should aim to:
- minimize complications by ensuring that dressings can be cleanly removed
- avoid delays in healing by maintaining healthy periwound skin
- optimize patient comfort during wear
The DURAFIBER range is a new generation of high-performing gelling fiber dressings for medium to highly exuding wounds.
Strength in Performance
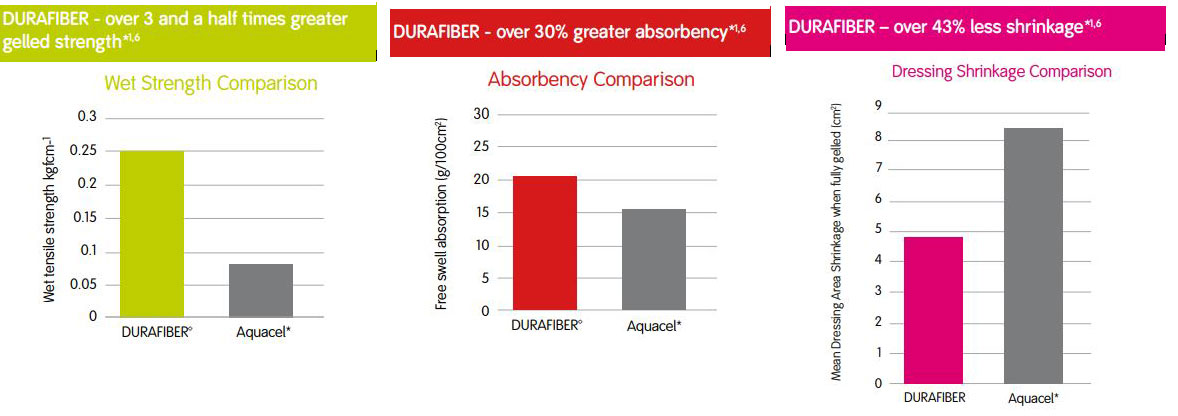
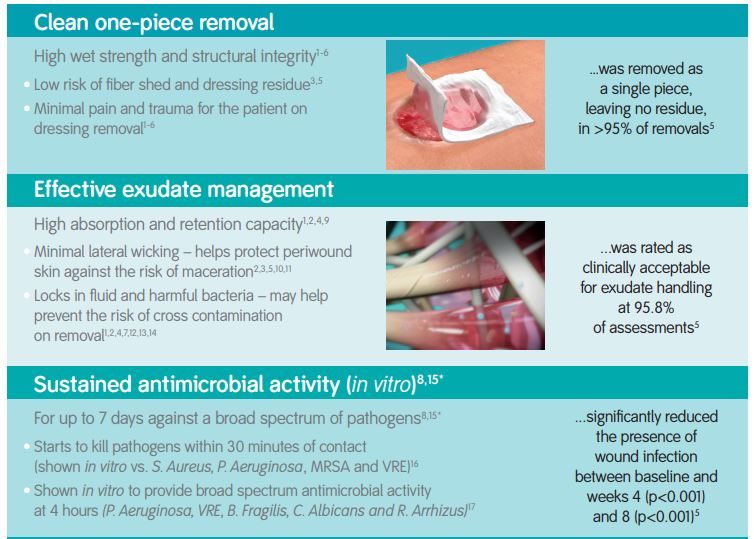
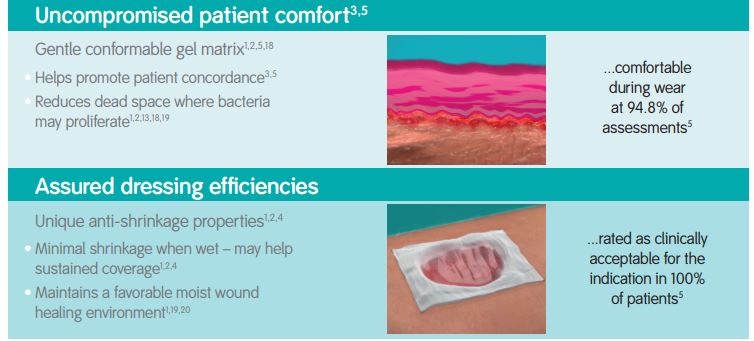
Indications
DURAFIBER is indicated as an absorbent, gelling dressing for the management of chronic and acute, full thickness, partial thickness, or shallow granulating exuding wounds. For example: leg ulcers; pressure ulcers; diabetic ulcers; surgical wounds; wounds left to heal by secondary intent; donor sites; tunneling and fistulae wounds; partial thickness burns; traumatic wounds; and wounds that are prone to bleeding such as wounds that have been surgically or mechanically debrided. DURAFIBER is designed to be left in place for up to 7 days.
Precautions
Whilst DURAFIBER assists in the management of wounds prone to bleeding, it is not intended to be used as a surgical sponge in heavily bleeding wounds. If reddening or sensitisation occurs discontinue use.
Instructions for Use
- Cleanse the wound according to local clinical protocol.
- Select the appropriate dressing size.
- Remove the DURAFIBER dressing from pack, using a clean technique. Cut to shape if necessary.
- Apply the dressing to the wound and allow for a 1cm / 1/3in. dressing overlap onto the skin surrounding the wound.
- When using DURAFIBER ribbon in deep cavity wounds, insert in one piece, leave at least 2.5cm / 1in. outside the wound for easy retrieval. Loosely pack deep wounds 85%, as the DURAFIBER dressing will expand to fill the wound dressing on contact with wound fluid.
- Secure DURAFIBER with a moisture retentive dressing (e.g. ALLEVYN) or other appropriate secondary retention.
- The dressing may adhere if used on lightly exuding wounds. If the dressing is not easily removed, moisten or soak the dressing to assist removal and avoid disruption of the healing wound.
- If DURAFIBER is used on infected wounds, the infection should be treated according to local clinical protocols.
Frequency of Dressing Change
During the early stages of wound management, DURAFIBER dressings should be inspected frequently. Dressings can be left undisturbed for up to 7 days or changed when clinically indicated, (e.g. if leakage, excessive bleeding is present). Local clinical protocol should also be taken into consideration.
DURAFIBER is a single use only product. If used on more than one patient, cross contamination or infection may result. Opening the dressing pack compromises the sterile barrier therefore any unused dressing should not be retained for application at a later date.
Dressing Removal
The dressing can be removed in one piece using sterile tweezers, forceps or a gloved hand. Ensure all dressings are removed.



