-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Hospira 07953-02 - LACTATED RINGERS INJECTION, USP, 250ML, EACH
07953-02 Lactated Ringer's Solution IV Solution Flexible Bag 250 mL, EACH
These products are sterile, nonpyrogenic solutions each containing isotonic concentrations of electrolytes (with or without dextrose) in water for injection. The solutions containing dextrose and electrolytes are hypertonic; those containing only electrolytes are isotonic. They are administered by intravenous infusion for parenteral replacement of extracellular losses of fluid and electrolytes, with or without minimal carbohydrate calories.
Lactated Ringer's IV Solution Components
Each 100 mL of Lactated Ringer's Injection, USP contains sodium chloride 600 mg, sodium lactate, anhydrous 310 mg, potassium chloride 30 mg and calcium chloride, dihydrate 20 mg. May contain hydrochloric acid and/or sodium hydroxide for pH adjustment. A liter provides 9 calories (from lactate), sodium (Na+), 130 mEq, potassium (K+) 4 mEq, calcium (Ca++) 3 mEq, chloride (Cl-) 109 mEq and lactate [CH3CH(OH) COO-] 28 mEq. The electrolyte content is isotonic (273 mOsmol/liter, calc.) in relation to the extracellular fluid (approx. 280 mOsmol/liter). The pH of the solution is 6.6 (6.0 - 7.5).
Each 100 mL of Lactated Ringer's and 5% Dextrose Injection, USP contains dextrose, hydrous 5 g plus the same ingredients and mEq values as Lactated Ringer's Injection, USP (contains only hydrochloric acid for pH adjustment). A liter provides 179 calories (from dextrose and lactate) and has a hypertonic osmolar concentration of 525 mOsmol (calc.). The pH is 4.9 (4.0 - 6.5).
The solutions contain no bacteriostat, antimicrobial agent or added buffer (except for pH adjustment) and each is intended only for use as a single-dose injection. When smaller doses are required the unused portion should be discarded. The solutions are parenteral fluid, nutrient and/or electrolyte replenishers. Dextrose, USP is chemically designated D-glucose, monohydrate (C6H12O6 H2O), a hexose sugar freely soluble in water. It has the following structural formula:
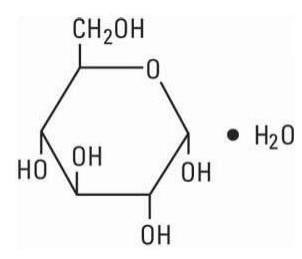
Calcium Chloride, USP is chemically designated calcium chloride, dihydrate (CaCl2 2 H2O), white fragments or granules freely soluble in water. Potassium Chloride, USP is chemically designated KCl, a white granular powder freely soluble in water. Sodium Chloride, USP is chemically designated NaCl, a white crystalline powder freely soluble in water. Sodium Lactate, USP is chemically designated monosodium lactate [CH3CH(OH)COONa], a 60% aqueous solution miscible in water. It has the following structural formula:
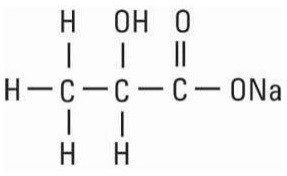
Water for Injection, USP is chemically designated H2O. The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions inside the plastic container also can leach out certain of their chemical components in very small amounts before the expiration period is attained. However, the safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers.
07953-02 Lactated Ringer's IV Solution Warnings
Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema with sodium retention. In patients with diminished renal function, administration of solutions containing sodium or potassium ions may result in sodium or potassium retention. Solutions containing lactate ions should be used with great care in patients with metabolic or respiratory alkalosis.
The administration of lactate ions should be done with great care where there is an increased level or an impaired utilization of lactate ions, as in severe hepatic insufficiency. The intravenous administration of these solutions can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
The risk of dilutional states is inversely proportional to the electrolyte concentrations of administered parenteral solutions. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations of such solutions.
Hospira Lactated Ringer's IV Solution Dosage and Administration
The dose is dependent upon the age, weight and clinical condition of the patient. As reported in the literature, the dosage and constant infusion rate of intravenous dextrose must be selected with caution in pediatric patients, particularly neonates and low birth weight infants, because of the increased risk of hyperglycemia/hypoglycemia.
Additives may be incompatible. Consult with pharmacist, if available. When introducing additives, use aseptic technique, mix thoroughly and do not store. The presence of calcium limits their compatibility with certain drugs that form precipitates of calcium salts, and also prohibits their simultaneous infusion through the same administration set as blood because of the likelihood of coagulation. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
07953-02 Hospira Adverse Reactions
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia. If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
- Manufacturer: 7953-02 ICU Medical
- Application: Replacement Preparation
- Container Type: Flexible Bag
- Dosage Form: IV Solution
- Generic Drug Name: Lactated Ringer's Solution
- Type: Intravenous
- Volume: 500mL
- Latex Free Indicator: Not Made with Natural Rubber Latex
- MADE IN USA



