-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

ICU Medical MC100 - MicroClave Clear I.V. Connector Swabable Seal Surface, FR 165 mL/min, Dead Space 0.04 mL, Backpressure +45 PSIG. Non-DEHP/Latex, 100/Ca
MICROCLAVE CLEAR NEEDLEFREE NEUTRAL DISPLACEMENT CONNECTOR.
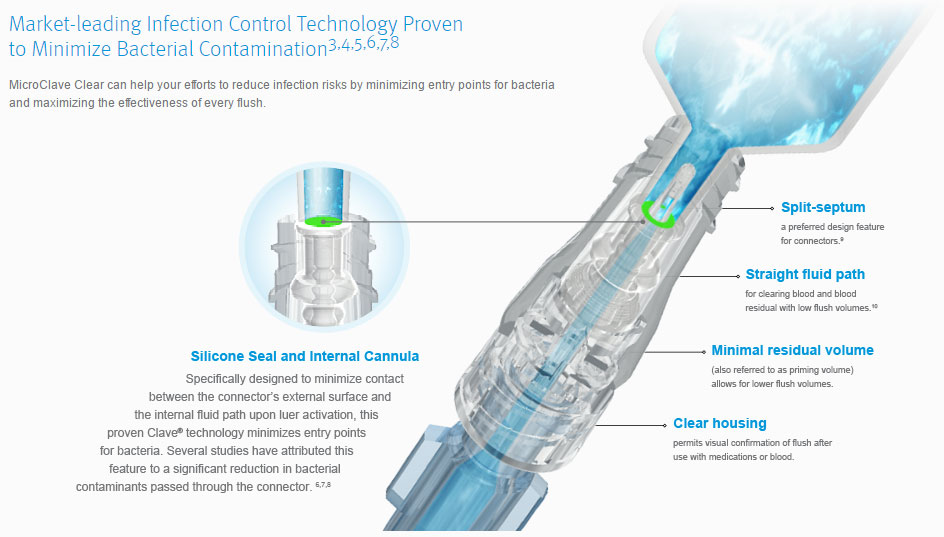
MicroClave is a clear housing, needlefree device with neutral displacement straight fluid path design, split-septum and minimal dead space that will help protect your patients and clinical staff by providing a safe and effective microbial barrier and will also help visualize connector flushing.
The MicroClave Clear is the only neutral displacement connector that combines market-leading Clave technology with a clear housing that lets you visualize the fluid path, so you can clearly see whether you have completely flushed the connector after blood draws or administration.
The design of your needlefree intravenous (I.V.) connectors plays a substantial role in your ability to limit hospital-acquired bloodstream infections (HA-BSI). Not only does the MicroClave Clear provide enhanced patient safety through innovative technology, but it has also been proven to provide an effective microbial barrier against bacteria transfer and contamination.
Additional Features:
The MicroClave Clear can be used on all peripheral, arterial, and central venous catheters for the administration of IV fluids or medications, and can be used with blood products. Does not require a change in clinical practice or technique and may help you address recent concerns raised by the FDA regarding the safety of positive displacement connectors.
HOW IT WORKS
Unique features of the MicroClave Clear that may help reduce the risk of bacterial contamination:
- Split-septum is noted in the CDC guidelines as a preferred design feature for connectors.
- Straight fluid path allows for clearing of blood and blood residual with low flush volumes.
- Minimal dead space (also referred to as residual volume) of 0.04 mL allows for lower flush volumes.
- Clear housing permits visual confirmation of flush after use with medications or blood.
- Allows a saline flush option, which can eliminate the risk of Heparin Induced Thrombocytopenia (HIT).
- Clamping sequence not required, reducing educational burden and risk of error.
- Approved for use with power injectors.
- Allows you to effectively clear blood from the connector with as little as a 2.5 mL saline flush.
Accessing the Fluid Path
- When MicroClave Clear is not being accessed, the silicone seal forms a safe, swabbable barrier to bacterial ingress.
- Upon luer access, the silicone seal is depressed and the fluid path windows are exposed through the device's split septum.
- Fluid is either infused or aspirated through the connector and catheter via the dedicated straight internal fluid path.



