-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Lifesign 20935 - Status DS Tricyclic Antidepressant (TCA) (35 Tests)
Status DS TCA
One Step Immunoassay For The Qualitative Detection Of Drugs Of Abuse And Their Metabolites In Urine.
- Simple one-step procedure
- Accurate results with a 97-99% correlation to GC/MS
- Fast turnaround time with results in 5-10 minutes
- Flexible combination of drugs from a single drug up to 10 drug tests
Status DS TCA Intended Use
Status DS TCA is a simple, one-step immunochromatographic assay for the rapid, qualitative detection of tricyclic antidepressants (TCAs) . The test is standardized to detect nortryptiline at a cutoff concentration of 1000 ng/mL in human urine.
The Status DS TCA test is a qualitative screening test, and provides only a preliminary analytical result. A negative result does not eliminate the possibility of the presence of tricyclic antidepressants (TCAs) in the urine specimen at concentrations below the cutoff. A positive result may be due to the sum of the reactivities of more than one tricyclic antidepressant and/or their metabolites (see Table 2: Specificity). To obtain a confirmed analytical result, a more specific alternative method should be used, e.g., high performance liquid chromatography (HPLC) or gas chromatography, mass spectrometry (GC/MS). Clinical consideration and professional judgment should be applied to any drug test result, particularly when preliminary positive results are used.
Summary and Explanation of Status DS TCA
Tricyclic antidepressants (TCAs) are a type of prescription drug intended for clinically depressed patients. Unfortunately, they are becoming more frequently abused and are now one of the leading causes of death by drug overdose in the United States. There are two broad chemical classes of TCAs. The tertiary amines - amitryptiline, imipramine, trimipramine and doxepin - boost serotonin levels and are prescribed for insomnia, irritability and overstimulation. The secondary amines - nortryptiline, desipramine and protryptiline - enhance nore- pinephrine levels and are prescribed for opposite types of symptoms, such as excessive fatigue, withdrawal and inertness. Abuse of TCAs may lead to coma, respiratory depression, convulsions, blood pressure deviations, hyperprexia and severe cardiac conditions. TCAs are excreted in urine mostly in the form of metabolites for up to ten days.
Principle of Status DS TCA
The Status DS TCA test uses solid-phase chromatographic membrane immunoassay technology for the qualitative detection of tricyclic antidepressants. The test is based on the principle of the highly specific immunochemical reactions between antigens and antibodies which are used for the analysis of specific substances in biological fluids. The test relies on the competition between the drug conjugates and the drugs which may be present in the urine sample, for binding to antibodies. In the test procedure, a sample of urine is placed in the Sample Well of the device and is allowed to migrate upward. If the drug is present in the urine sample, it competes with the drug conjugate bound to the dye, for the limited antibodies immobilized on the membrane. If the level of drug or drug metabolite is above the cutoff level, the drug will saturate the antibodies, thus inhibiting the binding of the dye coated with drug conjugates to the antibodies on the membrane.
This prevents the formation of a line on the membrane. Therefore, a drug-positive urine sample will not generate a line at Test (T) position in the Result Window, indicating a positive result from positive drug competition. A negative urine sample will generate a line at T position in the Result window, indicating a negative result from an absence of competition with free drugs.In addition to the Test line that may appear in the Result window, a Control line is present to confirm the viability of the test. This Control line (validation line) should always appear if the test is conducted properly. Polyclonal sheep anti-mouse IgG antibody is immobilized on the control line. The monoclonal antibody-dye conjugates that pass the line will be captured and produce a colored line at the Control position (C). This works as a procedural control, confirming that proper sample volume was used and the reagent system at the Control line and the conjugate-color indicator worked properly. If insufficient sample volume is used, there may not be a Control line, indicating the test is invalid.
Materials Provided
The Status DS TCA test kit contains all the reagents necessary to perform the tests.
- Status DS TCA device. The test device contains a membrane strip coated with polyclonal anti-nortryptiline antibody and a pad containing drug-dye conjugate in a protein matrix.
- Disposable specimen pipette.
- Instructions for use.
Test Procedure
The test procedure consists of adding the urine sample to the Sample Well of the device and watching for the appearance of colored lines in the Result Window.
Test Protocol
For each test, open one Status DS pouch and label the Status DS device with the patient ID.
Holding the pipette vertically, dispense 3 full drops (110 L) of the urine sample into the Sample Well.
Interpretation of Results
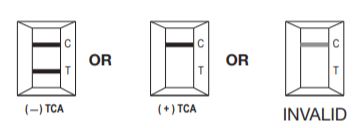
Negative: The appearance of a reddish-purple Control line (C) and a line for a specific drug indicates a negative test result; i.e., no drug above the cutoff level has been detected. The color intensities of the Control line and a specific drug line may not be equal. Any faint line in the Result window, visible in 10 minutes, should be interpreted as negative. A negative test result does not indicate the absence of drug in the sample; it only indicates the sample does not contain drug above the cutoff level in qualitative terms.
Positive: The appearance of a reddish-purple Control line and no distinct line next to a specific drug name indicates the test result is positive for that drug (i.e., the specimen contains the drug at a concentration above the cutoff level). A positive test result does not provide any indication of the level of intoxication or urinary concentration of the drug in the sample; it only indicates the sample contains drug above the cutoff level in qualitative terms.
Expected Values
Status DS TCA is a qualitative assay. The amount of nortryptiline present in the urine cannot be estimated by the assay. The assay results distinguish positive from negative samples. Positive results indicate the samples contain nortryptiline above the cutoff concentration.
Performance Characteristics
Accuracy: Comparison of Status DS TCA with Triage
| Triage | |||||
| Positive | Negative | TOTAL | |||
| Status DS (TSA) | Positive | 103 | 2 | 105 | |
| Negative | 0 | 98 | 98 | ||
| TOTAL | 103 | 100 | 203 | ||
Precision and Accuracy
The precision of the Status DS TCA assay was determined by carrying out the test with serially diluted standard drug solutions. About 95% of the samples containing nortryptiline concentrations 25% over the cutoff level consistently showed positive results. The study also included over 40 samples (+/-) 25% cutoff level. These results were found to be consistently in agreement with expected test results.
Distribution of Random Error:
Twenty (20) blind samples prepared by spiking various concentrations of nortryptiline were separately tested by two operators. The test results from the two operators showed complete agreement.


