-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Lifesign 33225 - Status Covid-19/Flu A&B (25 Tests)
Status Covid-19/Flu A&B
A Rapid Immunoassay for the Simultaneous Direct Detection and Differential Diagnosis of SARS-CoV-2, Influenza Type A and Type B Antigen from anterior nasal and nasopharyngeal swab specimens.
- COVID-19
- Anterior nasal swab specimen - Sensitivity 93.8 %, Specificity 100%
- Nasopharyngeal - Sensitivity 93.1 %, Specificity 100%
- Flu A - Sensitivity 91.4%, Specificity 95.7%
- Flu B - Sensitivity 87.6%, Specificity 95.9%
- FDA Emergency Use Authorization (EUA)
- Visually read in 15 minutes
- Flocked nasopharyngeal swab for superior specimen collection and patient comfort
Status COVID-19/Flu A&B test is a lateral flow immunoassay intended for the in vitro rapid, simultaneous qualitative detection and differentiation of nucleocapsid antigen from SARS-CoV-2, influenza A and/or influenza B directly from anterior nasal or nasopharyngeal swab specimens obtained from individuals, who are suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider, within the first five days of onset of symptoms. Clinical signs and symptoms of respiratory viral infection due to SARS-CoV-2 and influenza can be similar. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. 263a, that meet the requirements to perform moderate, high, or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
Results are for the simultaneous identification of nucleocapsid antigens of SARS-CoV-2, influenza A and influenza B, but does not differentiate between SARS-CoV and SARS-CoV-2 viruses and is not intended to detect influenza C antigens. These viral antigens are generally detectable in anterior nasal or nasopharyngeal swab specimens during the acute phase of infection. Positive results indicate the presence of viral antigens, but the clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or coinfection with other viruses. The agent detected may not be the definite cause of the disease. Laboratories within the United States and its territories are required to report all SARS-CoV-2 results to the appropriate public health authorities.
Negative SARS-CoV-2 results should be treated as presumptive and confirmed with a molecular assay, if necessary, for patient management. Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patients recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID19.
Negative influenza A and B test results should be treated as presumptive. It is recommended these results be confirmed by viral culture or an FDA-cleared influenza A and B molecular assay. Negative results do not preclude influenza virus infection and should not be used as the sole basis for treatment or other management decisions.
Performance characteristics for influenza A and B were established during the 2007-2009 and the 2014-2016 influenza seasons when influenza A/H1N1, A/H1N1 pandemic, A/H3N2, influenza B/Victoria lineage, and B/Yamagata lineage were the predominant influenza viruses in circulation according to the Flu Activity & Surveillance reports from the CDC. When other influenza viruses are emerging, performance characteristics may vary.
The performance of this test for SARS-CoV-2 was established based on the evaluation of a limited number of clinical specimens collected between September 2020 and April 2021. The clinical performance has not been established in all circulating variants but is anticipated to be reflective of the prevalent variants in circulation at the time and location of the clinical evaluation. Performance at the time of testing may vary depending on the variants circulating, including newly emerging strains of SARS-CoV-2 and their prevalence, which change over time.
The Status COVID-19/Flu A&B test is intended for use by medical professionals and laboratory personnel trained to perform the test. The Status COVID-19/Flu A&B test is only for use under the Food and Drug Administrations Emergency Use Authorization.
Status COVID-19/Flu A&B (Anterior Nasal or Nasopharyngeal Swab Specimens)
Study the Package Insert thoroughly before using Quick Reference Instructions.
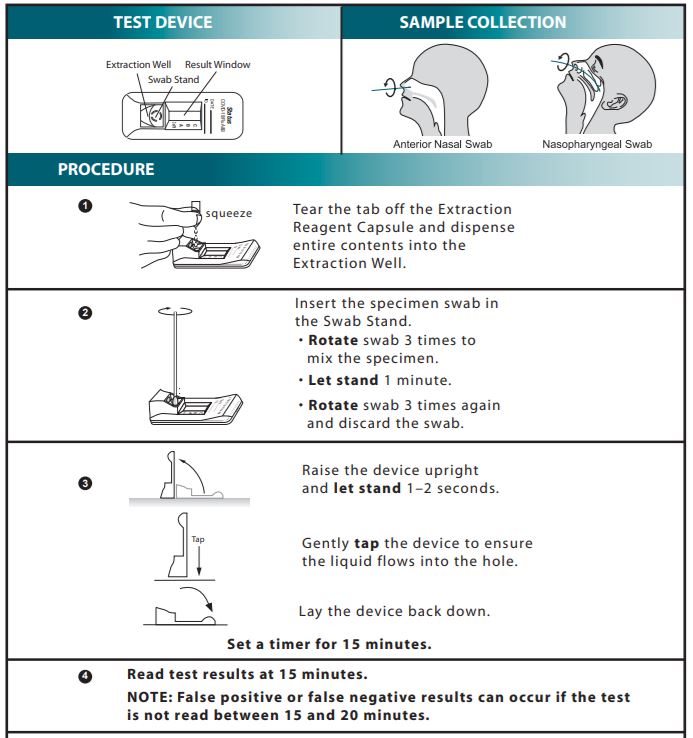
- For use under the Emergency Use Authorization (EUA) only
- For in vitro diagnostic use
- Rx only
- Refer to the Package Insert for complete instructions. Read the complete test procedure, including recommended Quality Control procedures, before performing the test.
- All clinical specimens must be at room temperature before beginning the assay.
- Performing the assay outside the time and temperature ranges provided may produce invalid results.
- Assays not performed within the established time and temperature ranges must be repeated.
- Expiration date: Check expiration on each individual test package or outer box before using. Do not use any test past the expiration date on the label.
INTERPRETATION OF RESULTS
Positive: At (15) minutes, the appearance of a reddish purple Control line (C position) and a reddish purple Test line (Flu A, Flu B or CoV19 position) indicate that Influenza A, B and/or SARS-CoV-2 antigen has been detected. Lines at the A and C positions indicate the presence of Influenza type A viral antigen, lines at the B and C positions indicate the presence of Influenza type B viral antigen, and lines at the CoV19 and C positions indicate the presence of SARS-CoV-2 viral antigen in the specimen. A positive result does not rule out co-infections with other pathogens or identify any specific influenza A virus subtype.
Negative: A reddish purple Control line (C position) only, with no Test line at the A, B, CoV19 positions, indicates that Influenza A, B antigen or SARS-CoV-2 antigen has not been detected. A negative result does not exclude influenza viral or SARS-CoV-2 viral infection. Determination of negative results should not be made before 15 minutes.
Negative Results are presumptive and may need to be confirmed with a molecular assay.