-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Lifesign 37030 - Status H. pylori, 30/BX
Status H. pylori
For the Qualitative Detection of Anti-Helicobacter pylori Antibody in Human Whole Blood, Serum or Plasma.
Status H. pylori qualitatively detects anti-Helicobacter pylori antibody in human whole blood, serum, or plasma specimens. The test is intended for use as an aid in the diagnosis of H. pylori infection in adult patients with symptoms of gastrointestinal disorders.
- CLIA WAIVED for whole blood
- Fingerstick whole blood convenience
- Requires only 25 l of whole blood or 10 l serum/plasma
- Single-reagent procedure
- Results available in 10 minutes
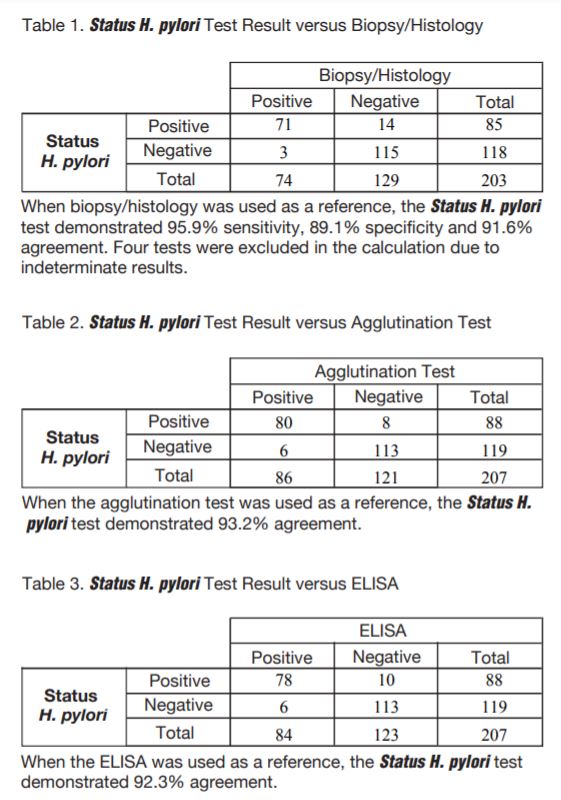
- Sensitivity of 95.9% with 91.6% overall agreement to biopsy histology
Summary & Explanation
Helicobacter pylori, formerly known as Campylobacter pylori, are gram-negative microaerophilic spiral bacteria that have been identified and cultured since 1983 1 .They can colonize the gastric mucosa for years 2 , and their presence is strongly associated with chronic, diffuse, superficial gastritis of the fundus and antrum 3- 5. As a result, they are now believed to have an etiologic role in gastritis 6,7. Recent evidence suggests that H. pylori gastritis may progress over several decades to chronic atrophic (type B) gastritis 8,9, a lesion that is a precursor of gastric carcinoma. The epidemiologic features of gastric carcinoma and H. pylori infection are similar 10, and recent studies suggest that H. pylori infection may be a risk factor for gastric carcinoma 11,12.Until recently, diagnosis of infection with H. pylori required endoscopy and identification of the organism by means of subsequent culture of the bacteria and/or recognition of spiral organisms in histologically evaluated sections of gastric tissue. However, the expense and invasive nature of this procedure make endoscopy impractical for epidemiologic studies. Serology has become the method of choice for such studies.There is excellent correlation between a classical clinical presentation of gastritis, the presence of H. pylori in the stomach and elevated serum levels of anti-H. pylori antibodies 13-15. Positive results can justify a short empirical trial of antimicrobial therapy in gastritis of unknown origin, and response to treatment can be serially monitored because levels of H. pylori-specific antibodies can be expected to fall significantly after successful antibacterial therapy.
Principle
The Status H. pylori - One-Step Anti-H. pylori Antibody Test utilizes indirect solid-phase immunoassay technology for the qualitative detection of H. pylori antibodies. Status H. pylori consists of H. pylori antigen on the test membrane and H. pylori antigen plus anti-human immunoglobulin antibodies coated on gold particles in the dye pad. Thus, in principle, the results of Status H. pylori may differ from the results of assay using only anti-IgG as a detector. In the test procedure, patient specimen is added in the upper area of the Sample well (S) located below the Result window.
The Developer solution is then added in the Sample well. The solution mobilizes the dye conjugated to H. pylori antigen and to anti-human immunoglobulin antibodies. If any anti-H. pylori antibody is present in the sample, the dye conjugate will bind to the H. pylori antigen band impregnated on the test membrane. Visualization of the antigen band at the Test position (T) will occur only when the anti-H. pylori antibody is present in the sample. As the antibody-dye conjugate continues to move along the test membrane, it will be captured by a species specific antibody located at the Control position (C) to generate a colored band regardless of the presence of H. pylori antibodies in the sample. The presence of two colored bands, one at the Test position and the other at the Control position, indicates a positive result, while the absence of a colored band at the Test position indicates a negative result.
Test Procedure
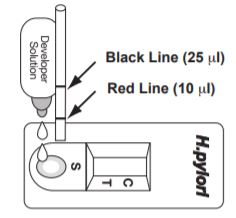
STEP 1 Remove a device from pouch and place on flat surface.
STEP 2 For serum or plasma fill a capillary tube to the red line (10 l). For whole blood fill a capillary tube to the black line (25 l). Apply sample by lightly tapping the capillary on the pad of the UPPER AREA of the Sample well (S).
STEP 3 Add 2 to 3 drops of Developer Solution onto the LOWER AREA of the Sample Well (S). STEP 4 Read result at 10 minutes. (Do not read after 15 minutes).
Interpretation of Results
Positive
One colored band each at the Test position (T) and at the Control position (C) indicates that antibodies against H. pylori have been detected.
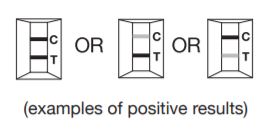
NOTE: The test result can be read as soon as a distinct pink-purple colored Test line (T) and a colored Control line (C) appear. Any shade of pink-purple colored Test line should be reported as a positive result. Possible positive results :
a. Two strong colored lines at both the Test (T) and Control (C) position.
b. One strong Test line (T) and one light colored Control line(C).
c. One light colored Test line (T) and one strong colored Control line (C).
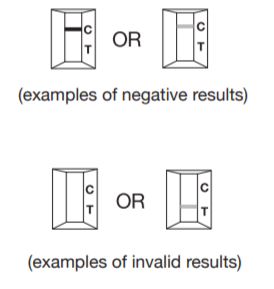
Negative
Only one colored Control line (C), with no colored Test line (T) indicates that antibodies against Helicobacter pylori have not been detected.
Invalid
A distinctive colored Control line (C) should always appear. The test is invalid if no Control line forms. Repeat the test with a new Status H. pylori test.

Lifesign #200013, Status H. Pylori Control Neg/Pos Ea
$83.70 per EACH

Lifesign #37030, Status H. Pylori, Whole Blood-Plasma/Serum, CLIA Waived/Moderate, 30 tests/bx (Item is Non-Returnable)
$160.00 per BOX

Lifesign #200013, Status H. Pylori Control, 1 ml, 2/bx
$91.84 per BOX

