By Category
-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
By Brand
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Part Number
Medline CRR101053
SKU Number
CIA1139155
Sell Unit
BOX
Ships Within
24 Hours
List Price
Call for Pricing
Product Description
Medline CRR101053 - DRESSING, CARRADRES, CLEAR, SHEET, 4"X4", 10 EA/BX, 6 BX/CS
CarraDres Clear Hydrogel Sheets
A sterile, hydrogel polymer sheet consisting of 89.5% water in a 10.5% cross-linked polyethylene oxide matrix. It is backed with an inert low density film that controls water vapor transmission during storage and makes the gel easy to handle. It has a high specific heat to provide a cooling effect, is hydrophilic, and will absorb three times its weight in fluid. Its clear to allow visualization of the wound and has gentle adhesion for sensitive peri-wound skin.
- Composed of 89.5% water and 10.5% polyethylene oxide, these sterile hydrogel sheets cool the affected area on contact and absorb 3 times their weight in fluid.
- Transparency allows for easy wound evaluation.
- The dressings are comfortable and gentle on the wound and surrounding tissue.
- RadiaDress is specifically designed for use in the management of 1st and 2nd degree burns, including radiation reactions.
About Hydrogels
- Donates moisture
- Rinses easily from the wound.
- Skintegrity 1-oz. bellows bottle reduces waste and eases application.
Recommended Use
- All wound depths.
- No/minimal drainage.
- As a primary dressing.
Recommended Secondary Dressing
- Stratasorb Composite.
- Bordered gauze.
- Suresite 123+Pad.
Indications
- Pressure injuries.
- Partial- and full-thickness wounds.
- Leg ulcers.
- Surgical wounds.
- Lacerations, abrasions and skin tears.
- First- and second-degree burns.
Change Frequency
- Skintegrity may be left in place for up to 3 days.
- Dressing change frequency will depend on amount of drainage.
Contraindication
- Patients with a known sensitivity to components of the gel.
- Heavily draining wounds.
Cytotoxicity Test For Skin tegrity Hydrogel
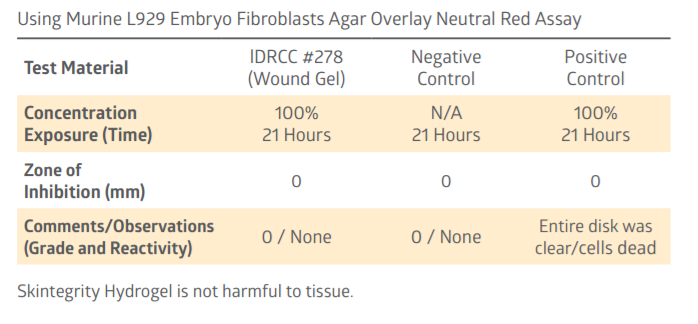
Technical Safety Data Sheet
1. Ingredients

2. First-Aid Measures
- Eye Contact:Flush eyes with large amounts of water for at least 15 minutes. Remove contact lenses, if worn. If irritation persists, seek medical attention.
- Skin Contact:If irritation develops wash area with water. Get medical attention if irritation persists.
- Inhalation:Remove victim to fresh air and keep at rest in a position comfortable for breathing. Seek medical attention if discomfort continues or if you feel unwell.
- Ingestion:Never give anything by mouth to an unconscious person. Consult a physician if necessary.
3. Accidental Release Measures
- Non-hazardous Small Spill:Should be cleaned up at the time of the spill. Take all necessary precautions and wear any personal protective equipment that is applicable. Dispose of per local and state regulations.
- Non-hazardous Large Spill:Should be cleaned up at the time of the spill. May require special treatment, equipment and/or emergency assistance. Dispose of per local and state regulations.
4. Exposure Controls/Personal Protection
- Engineering Controls:No specific measures are required provided the product is handled in accordance with the general rules of occupational hygiene and safety. Use in a well ventilated area to prevent exposure. Maintain eyewash fountain and quick-drench facilities in work areas.
- Respiratory Protection:None required under normal use conditions. Skin Protection: None required for normal use. For prolonged exposure, use appropriate goggles, protective clothing and gloves.
- Eye Protection:None required for normal use. For prolonged exposure, use appropriate goggles, protective clothing and gloves. Other protective equipment: Not expected to be necessary under normal conditions of use. Where exposure cannot be adequately controlled, use appropriate protective clothing or equipment
- Work/hygienic practices:Handle in accordance with good industrial hygiene and safety practice. Wash thoroughly with soap and water after handling and before eating, drinking, or using tobacco. Safety shower and eye wash should be available close to work areas.
5. Physical and Chemical Properties
- Physical State Sheet of gel
- Color Clear,
- Colorless
- Odor Odorless
- Odor Threshold N.D.
- Solubility 10%
- Partition coefficient Water/n-octanol N.D.
- VOC% N/A
- Viscosity N.D.
- Specific Gravity 0.014
- Density lbs/Gal N/A
- Pounds per Cubic Foot N/A
- Flash Point N.E.
- FP Method N.E.
- Ph 6.5-9.5
- Melting Point N.A.
- Boiling Point N.A.
- Boiling Range N.D.
- LEL N/A
- UEL N/A
- Evaporation Rate N.D.
- Flammability N.E.
- Decomposition Temperature N.D.
- Auto-ignition Temperature N.D.
- Vapor Pressure N.A.
- Vapor Density N.A.
Specifications
- Dressing Change Frequency/Use Max: 3 Day: As Needed.
- Hydrogel Sheet.
- HPIS Code 740_110_20_0
- Latex Free Yes.
- Length Inches 4 in
- Primary Dress.
- Product Shape Square
- Sterile Yes
- UNSPSC 42311515
- Width Inches 4 in
- Wound Condition Type Shallow/Deep,All Drainage Type.
Related Products



