-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Sol-Millennium Medical 100018IM - SOL-CARE 1ml TB Safety Syringe w/Fixed Needle 25G*5/8 1000/Case
Sol-Millennium 100018IM Sol-Care Safety Syringe with Fixed Needle
The Sol-Care Safety Syringes are equipped with a fixed needle, preventing reuse and lowering the risk of needlestick injuries.
The safety mechanism is activated when the user pulls the needle into the barrel of the syringe after medication delivery. This action covers the needle and locks prevention of needlestick injuries with a manual retractable needle mechanism.
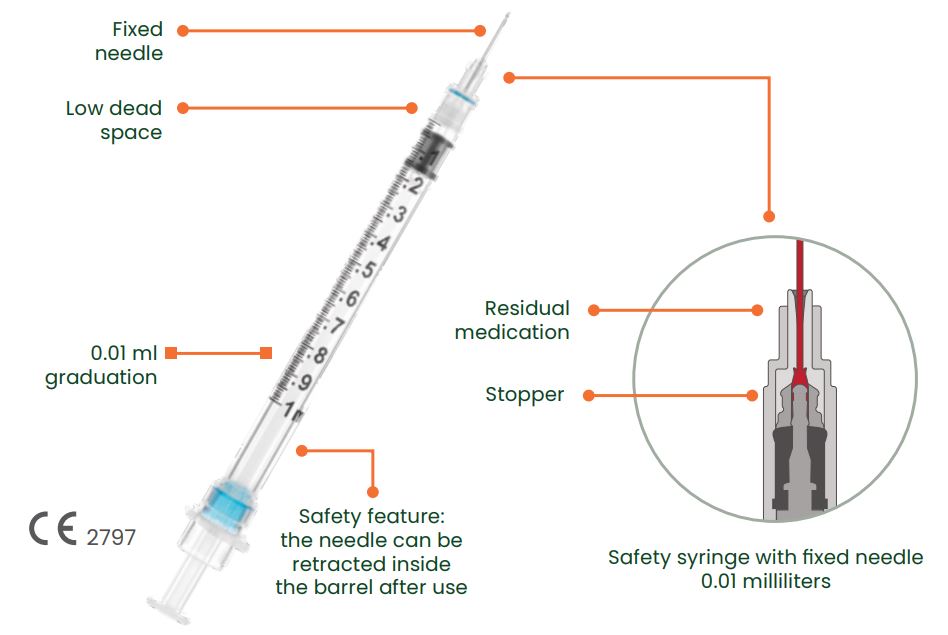
- Low Dead Space design (0.01mL) reduces waste and allows for more efficient medication delivery
- Convenient injection solution for key therapeutic areas, such as allergy and immunization
- Clear, bold graduations for accurate dosing
- No Gap design allows the plunger to be pushed to the zero mark without activating the safety mechanism
- Visual and audible confirmation of safety mechanism activation
- Patented locking ring design the syringe cannot be reused once the needle is locked within the barrel
| Reference number | Capacity | Gauge size | Outer diameter (mm) | Length (mm) | Length (inch) | Shelf Box / Shipping Case (pcs) |
| 100018IM | 1 ml | 25G | 0.5 mm | 16 mm | 5/8" | 100/1000 |
The syringe Sol-Care Safety Syringe with Fixed Needle has been designed to reduce the medication waste by reducing the dead space. It features a retracting needle design which helps to prevent the risk of needlestick injuries.
- Designed according to ISO 7886-1 and ISO 23908
- Not manufactured with Latex, PVC and DEHP
Intended Use of Sol-Millennium 100018IM Sol-Care Safety Syringe with Fixed Needle
Used to inject fluids into, or withdraw fluids from, the body. In addition, the Safety Syringe is designed to aid in the prevention of needle stick injuries.
Intended user: Licensed healthcare professionals (HCP).
Cautions
- Consult instruction for use before using this device
- Users must be trained by a healthcare professional and follow the Instructions
- For Use. Failure to follow these instructions could result in serious injury to thepatient and/or clinician.
- Sharp object. Risk of sharp injuries.

Safety For Clinicians
- Fingers remain behind the needle at all times.
- Visual and audible confirmation of safety mechanism activation.
- The second audible click confirms needle completely retracted and securely encased.
- Syringe cannot be reused once plunger permanently.
- locked within the barrel.
Easy to Use
- Minimal change in technique.
- Safety mechanism does not obstruct the view of the needles tip.
- Clear bold graduations for accurate dosing.
Patient Care & Efficiency
- Product allows the exchange of needle after drawing up of medication.
- Luer lock safety Syringe is intended to be used with standard hypodermic needles
Device Characteristics of Sol-Millennium 100018IM Sol-Care Safety Syringe with Fixed Needle
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |

Sol-Millennium Medical #100071IM, SOL-CARE 1ml Insulin Safety Syringe w/Fixed Needle 28G*1/2'' (U-100 Insulin Only) 1000/Case
$295.65 per CASE

Sol-Millennium Medical #100081IM, SOL-CARE 1ml Insulin Safety Syringe w/Fixed Needle 30G*1/2'' (U-100 Insulin Only) 1000/Case
$295.65 per CASE

Sol-Millennium Medical #100019IM, SOL-CARE 1ml TB Safety Syringe with Fixed Needle 27G x 1", 1000/Case
$300.45 per CASE

Sol-Millennium Medical #100034IM, SOL-CARE 1ml TB Safety Syringe with Fixed Needle 25G x 1" 1000/Case
$300.45 per CASE

