-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Sol-Millennium Medical SN3012 - Sol-Care Safety Needle, 30G x 1/12", Yellow, 800/CS
Sol-Millennium Medical SN3012 Sol-Care Safety Needle
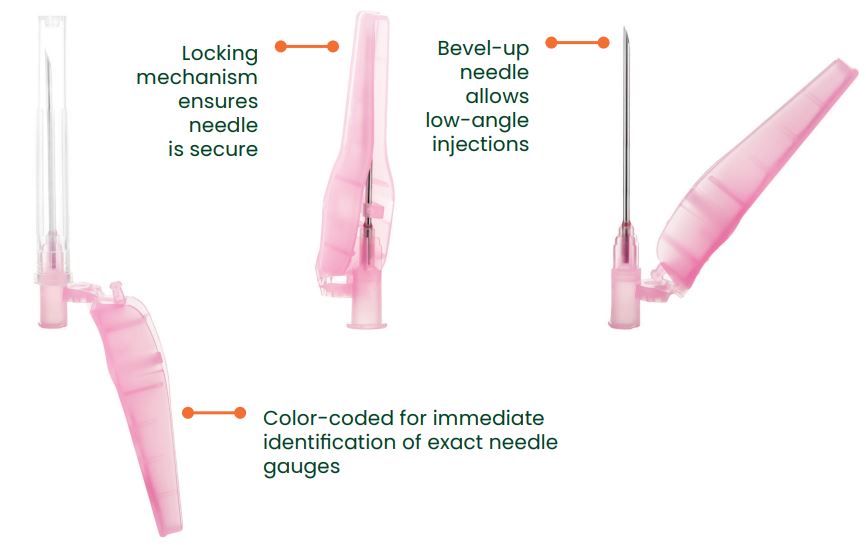
Sol-Care Safety Needles are designed to help prevent needlestick injuries. Every needle is equipped with a pivoting safety shield mechanism that locks in place to lower the risk of accidental injury after medication administration. There two methods of safety shield activation that keep hands and fingers behind and away from the needle tip
Sol-Millennium Medical SN3012 Sol-Care Safety Needle Features
- Two options for protective arm activation: hard surface activation and thumb activation
- Simple one-handed safety shield mechanism
- Audible click indicates safety mechanism is secure and locked in place
- Hubs, protective shields, and packaging are color-coded for immediate identification of needles gauges (ISO 6009 in compliance with criteria given by OSHA)
- Protective arm does not obstruct visualization of injection site
- Bevel-up needle allows for low-angle injections
- Compatible with all standard Luer Lock and Luer Slip Tip syringes
Two options for protective arm activation of Sol-Millennium Medical SN3012 Sol-Care Safety Needle

Device Characteristics of Sol-Millennium Medical SN3012 Sol-Care Safety Needle
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | Yes |
| For Single-Use: | Yes |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |

Sol-Millennium Medical #SN2515, Sol-Care Safety Needle, 25G x 1 1/12", Orange, 800/CS
$162.00 per CASE

Sol-Millennium Medical #SN2015, Sol-Care Safety Needle, 20G x 1 1/2, Yellow, 800/CS
$162.00 per CASE

Sol-Millennium Medical #13005SN, Sol-Care 1mL Luer Lock Syringe With Safety Needle, 30G x 1/2", Yellow, 300/CS
$126.00 per CASE

